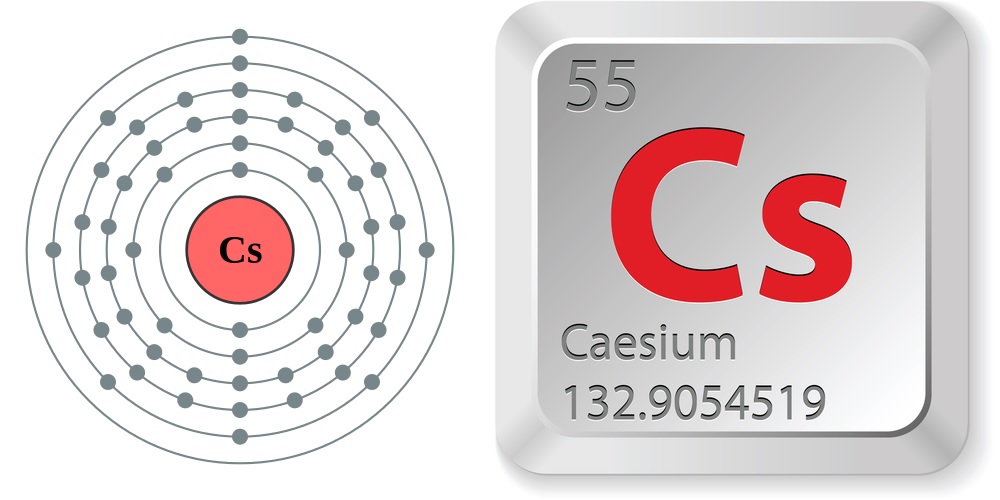
Facts About Cesium

Cesium is a rare, silver-white, shiny metal with brilliant blue spectral lines; the element's name comes from "caesius," a Latin word meaning "sky blue." It is the softest metal, with a consistency of wax at room temperature. It would melt in your hands — if it didn't explode first, as it is highly reactive to moisture.
Cesium is a naturally occurring element, although almost never on its own, according to the Jefferson Lab, with a presence in several minerals. It has a density of nearly twice that of water, and is very ductile.
Just the facts
- Atomic number (number of protons in the nucleus): 55
- Atomic symbol (on the periodic table of elements): Cs
- Atomic weight (average mass of the atom): 132.90
- Density: 1.086 ounces per cubic inch (1.879 grams per cubic centimeter)
- Phase at room temperature: solid
- Melting point: 83.1 degrees Fahrenheit (28.4 degrees Celsius)
- Boiling point: 1,239.8 F (671 C)
- Number of natural isotopes (atoms of the same element with a different number of neutrons): 1. There are also at least 39 artificial isotopes created in a lab.
- Most common isotopes: Cs-133 (100 percent of natural abundance)
History
Cesium was the first element to be discovered with a spectroscope. It was discovered in 1860 by German chemists Robert Bunsen and Gustav Kirchhoff when they were analyzing the spectrum of mineral water, according to WebElements.
The first practical applications of cesium were realized in the 1920s, according to the USGS. Cesium was used in vacuum tubes to remove traces of remaining oxygen due to its ready nature to bond with it, and as a coating on heated cathodes to increase the electric current. In later decades, more uses for cesium arose, including photoelectric cells, spectrometers and catalysts for organic reactions. The high cost of cesium and the growing popularity of similar and cheaper technologies using other alkali metals reduced the use of cesium to a handful of applications.
Who knew?
- Cesium is an alkali metal, along with lithium, sodium, potassium, rubidium and francium. Alkali metals are highly reactive, have one electron in their outer shell, and do not occur freely in nature, according to ChemicalElements. These metals are also very malleable, ductile and good heat and electrical conductors.
- Cesium is incredibly accurate at timekeeping and is used in atomic clocks. The official definition of a second is the time it takes for the cesium atom to vibrate 9,192,631,770 times between energy levels. Cesium-based atomic clocks lose one second per 100 million years.
- Cesium is one of four metals that are liquid at or near room temperature; the others are mercury (melting point of minus 37.9 F, or minus 38.8 C), gallium (melting point of 85.6 F, or 29.8 C) and francium (melting point of 80.6 F, or 27 C) according to Chemicool.
- According to Chemicool, the abundance of cesium in Earth's crust is about 3 parts per million by weight, and in the solar system 8 parts per billion by weight. Cesium is the 50th most common element in Earth’s crust, according to PeriodicTable.
- Cesium is highly reactive and combines readily with other elements, especially oxygen and other gases, and nonmetals, according to Encyclopedia Britannica.
- Because cesium reacts violently with water, it is treated as a hazardous material and is often kept under a layer of kerosene or mineral oil or in a vacuum to prevent it from reacting and igniting due to the moisture in the air. According to Encyclopedia.com, cesium also reacts violently with sulfur, phosphorous, acids, and halogens (fluorine, chlorine, bromine, iodine and astatine).
- Radioactive isotopes of cesium are produced in two ways: in nuclear power plants by the fission of uranium in fuel rods and by the explosion of nuclear weapons, according to the Agency for Toxic Substances and Disease Registry (ATSDR).
- Trace amounts of cesium are found in a handful of minerals, according to Minerals Education Coalition. It is mainly mined from pollucite (ranging from approximately 34 to 42 percent, according to the U.S. Geological Survey), as well as in lepidolite as an impurity. Cesium is mainly mined in Canada and Zimbabwe.
- According to the USGS, there are three ways to isolate cesium from the rest of the mineral: acid digestion (dissolving the minerals in acid and then separating the salts), alkaline digestion (roasting the mineral with calcium or sodium based mixture to extract cesium compounds then isolate the cesium salts), and direct reduction (heating the mineral ore with calcium, potassium, or sodium in a vacuum to separate an impure cesium metal).
- According to Lenntech, humans and animals are constantly exposed to trace amounts of cesium when eating, breathing and drinking. While it is unlikely that people will experience adverse health effects just due to the cesium, exposure to radioactive cesium or certain cesium compounds can lead to adverse health effects including nausea, vomiting, bleeding and cell damage.
Current research
Cesium is currently being researched in treatment of several forms of cancer, including brain tumors, according to a study published in 2016 in the journal Frontiers in Surgery. Cesium-131, a radioactive isotope of cesium, is used with iodine-125, another radioactive isotope, in a brachytherapy seed. According to the American Brachytherapy Society, a brachytherapy seed is a radioactive pod that is placed directly within the cancerous tissue. Brachytherapy seeds have been shown to be effective in several forms of cancer including prostrate, cervical, and endometrial cancers.
The above study refers to a previous study published in 2014 in the Journal of Neurosurgery. In the earlier study, a group of 24 patients with brain tumors had cesium-131 brachytherapy seeds implanted within the tumors. There were minimal side effects, and it was overall a well-tolerated form of treatment.
The idea for using cesium-131 brachytherapy seeds as a treatment for cancer dates back to the 1960s and was described in a study published in the journal Radiology. According to Isoray Medical, cesium-131 was FDA-approved in 2003 with the first trials in 2004. A 2009 study published in the Journal of Medical Physics, discusses using cesium-131 seeds for treatment in prostate cancer with positive results.
Get the world’s most fascinating discoveries delivered straight to your inbox.
And while more research is still needed to understand more about the treatment, all of the studies have shown that using cesium-131 brachytherapy seeds for cancer therapy is encouraging. The authors of the 2016 study state that they are confident that “future studies will likely confirm these promising preliminary results with cesium-based brachytherapy for future treatment of brain metastases.”
Additional resources

Rachel Ross is a science writer and editor focusing on astronomy, Earth science, physical science and math. She holds a Bachelor of Arts in Philosophy from the University of California Davis and a Master's degree in astronomy from James Cook University. She also has a certificate in science writing from Stanford University. Prior to becoming a science writer, Rachel worked at the Las Cumbres Observatory in California, where she specialized in education and outreach, supplemented with science research and telescope operations. While studying for her undergraduate degree, Rachel also taught an introduction to astronomy lab and worked with a research astronomer.