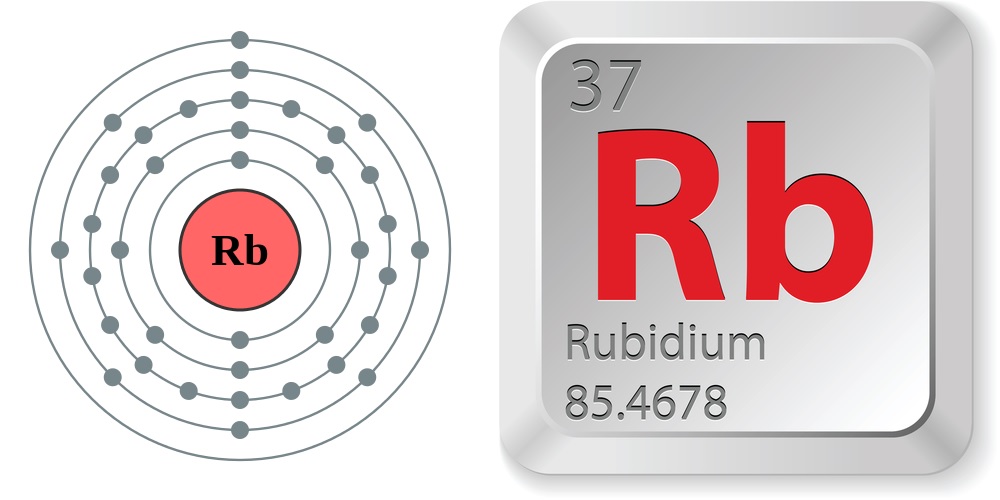
Facts About Rubidium

Rubidium is a silvery-white and very soft metal — and one of the most highly reactive elements on the periodic table. Rubidium has a density about one and a half times that of water and is solid at room temperature, although the metal will melt if it's just a bit warmer, according to Chemicool.
Like the other alkali metals (lithium, sodium, potassium, cesium and francium), rubidium reacts violently with water, oxidizes when reacting with oxygen, and ignites due to humidity in the air, so great care must be taken when working with the element. Scientists treat rubidium as a toxic element, according to Encyclopedia, although no known health effects of rubidium are known.
Just the facts
- Atomic number (number of protons in the nucleus): 37
- Atomic symbol (on the periodic table of elements): Rb
- Atomic weight (average mass of the atom): 85.4678
- Density: 0.886 ounces per cubic inch (1.532 grams per cubic cm)
- Phase at room temperature: solid
- Melting point: 102.7 degrees Fahrenheit (39.3 degrees Celsius)
- Boiling point: 1,270.4 F (688 C)
- Number of natural isotopes (atoms of the same element with a different number of neutrons): 2. There are also 29 artificial isotopes created in a lab.
- Most common isotopes: Rb-85 (72.2 percent of natural abundance), Rb-87 (27.8 percent of natural abundance)
History
Rubidium was discovered by German chemists Gustav Robert Kirchhoff and Robert Wilhelm Bunsen in 1861 when they were observing the spectrum of the mineral lepidolite as it burned, according to Peter van der Krogt, a Dutch historian. The spectrum of rubidium showed off two dark red lines, and the scientists named the newly discovered alkali metal rubidium after the Latin word for “deep red.”
According to Chemicool, rubidium was extracted from the surrounding mineral by electrolysis. Approximately 330 lbs. (150 kilograms) of the lepidolite ore was needed in order to extract enough rubidium (about 1.5 percent of the mineral) in order to study its properties. The scientists found that rubidium was more electropositive (meaning that rubidium tended to form positive ions more readily, according to Encyclopedia) than potassium, another alkali metal and reacted violently with water releasing hydrogen.
According to New World Encyclopedia, there was very little need or use for rubidium until the 1920s when it was used more frequently in research, chemical reactions, and electronic applications.
Who knew?
- Rubidium is the 25th most abundant element found on Earth, according to PeriodicTable. According to Chemicool, the abundance of rubidium in the Earth’s crust is 90 parts per million by weight and 30 parts per million by weight in the solar system.
- Alkali metals are highly reactive. They have one electron in their outer shell, and do not occur freely in nature, according to ChemicalElements. These metals are also very malleable, ductile, and good heat and electrical conductors.
- Rubidium forms alloys with other alkali metals as well as with elements such as mercury (known as amalgams) and gold, according to New World Encyclopedia. It also exists in many compounds that have uses several fields of study, such as rubidium chloride in biology and cell research.
- According to the Jefferson Lab, rubidium is used as a “getter” in vacuum tubes. Due to rubidium’s readiness to bond with several gases, it is used to remove any trace gases after the vacuum tubes are manufactured.
- According to Encyclopedia Britannica, it is difficult to extract pure rubidium due to its always being found with cesium and often times with other alkali metals. Separating the alkali metals is tricky due to the similar nature of many of the alkali metals.
- Rubidium ignites spontaneously when exposed to air and reacts violently with water, releasing hydrogen, which immediately bursts into flames. According to Encyclopedia Britannica, rubidium must be kept in mineral oil or in an inert gas atmosphere.
- According to the New World Encyclopedia, rubidium doesn’t have many commercial uses but it is used in vapor turbines, in vacuum tubes, in photocells, in atomic clocks, in some types of glass, the production of superoxide by burning oxygen, and with potassium ions in several biological uses.
- Rubidium and strontium are used to radioactively date rocks, minerals, and meteorites according to Encyclopedia Britannica. Rubidium-87 is an unstable isotope that has a half-life of about 50 billion years and decays to strontium-87, a stable isotope of strontium. Radioactive dating is performed by observing the ratio of strontium-87 to stronium-86, according to Encyclopedia.
- According to Chemicool, rubidium is used in fireworks to give the explosions a purple-red color.
- Due to the easy ionization of rubidium, it has been considered for use in ion engines for space-craft, according to the New World Encyclopedia. Cesium and xenon, however, prove to be more efficient.
- According to a 2015 study by Ruth H. Zadik, et al., a group of physicists and chemists from across Europe and Asia, published in Science Advances, rubidium was introduced into “bucky-balls” (carbon-60 molecules), rearranging the atoms into a new crystalline structure. The new structure showed a combination of properties including insulating, superconducting, metallic, magnetic, and what is known as a Jahn-Teller metal. A Jahn-Teller metal is a metal that can be transformed from an insulator into a conductor by applying pressure.
- Although a second is defined by the oscillations of a cesium atom, often times rubidium is used in atomic clocks, according to Periodic Table, due to a less expensive price tag. Several studies, including those done by ESA and Chinese researchers discuss using rubidium clocks on satellites, spacecraft, and on laser guiding systems where precise timing is needed.
Current research
A 2013 study by Adam Ghotbi, Andreas Kjær, and Philip Hasbak, Danish medical scientists, published in the Scandinavian Society of Clinical Physiology and Nuclear Medicine compares the use of rubidium-82, a radioactive isotope, as a tracer in use in PET technology with other methods and elemental tracers. According to the authors, rubidium-82 is becoming one of the most widely used elements in tracers in myocardial studies and procedures. With a half-life of 75 seconds, rubidium-82 is injected into the body and is used by the imaging system to detect damaged tissue and heart disease and, according to the authors, “yields excellent image quality.”
A similar study published in 2016 by Jennifer Renaud et al., a group of Canadian medical researchers, in the Journal of Nuclear Medicine. The study took place over a one-year period at three imaging centers with over 3,000 patients and was testing the quality of imaging using rubidium-82. The authors concluded that the use of rubidium-82 in PET scans was “accurate and precise, with consistent performance between different units and imaging centers.”
Get the world’s most fascinating discoveries delivered straight to your inbox.
Using rubidium-82 for medical purposes dates back to 1954, according to a 2015 study by Jean-François Chatal, et al., a group of French medical researchers, published in Frontiers in Medicine in the Nuclear Medicine subsection. The FDA approval to use rubidium-82 in myocardial treatments came in 1989. The authors summarize the pros and cons of rubidium-82 which include low radiation exposure and a with high quality images as the pros and cost and a limited number of available PET systems being the cons. Even with the cons, the use of rubidium-82 is ideal in myocardial studies.
Additional resources

Rachel Ross is a science writer and editor focusing on astronomy, Earth science, physical science and math. She holds a Bachelor of Arts in Philosophy from the University of California Davis and a Master's degree in astronomy from James Cook University. She also has a certificate in science writing from Stanford University. Prior to becoming a science writer, Rachel worked at the Las Cumbres Observatory in California, where she specialized in education and outreach, supplemented with science research and telescope operations. While studying for her undergraduate degree, Rachel also taught an introduction to astronomy lab and worked with a research astronomer.