Facts About Arsenic

From the time of the Roman Empire all the way to the Victorian era, arsenic was considered the "king of poisons" as well as the "poison of kings." History is riddled with accounts of both royalty and commoners carrying out assassinations for personal gain using the odorless, tasteless — in other words, poison-perfect — compounds of arsenic.
But even with its reputation as a lethal substance, arsenic still holds a very important place in the natural world.
A natural chemical
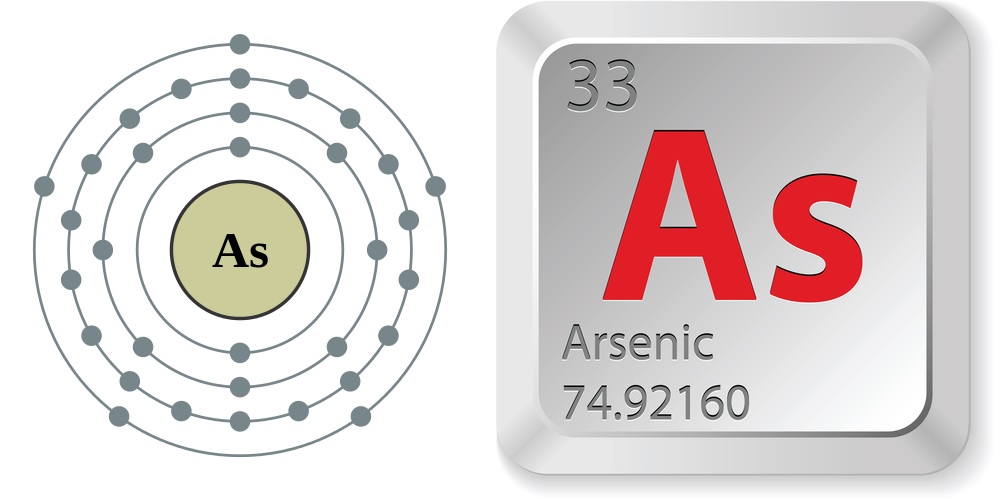
In the periodic table of the elements, arsenic is No. 33. An arsenic atom has 33 electrons and 33 protons with five valence electrons (those that can participate in forming chemical bonds with other electrons) in its outer shell.
Arsenic is a crystalline metalloid found in the Earth's crust, but in its free form it is quite rare. The element is typically found in minerals, such as arsenopyrite, realgar and orpiment, according to the Minerals Education Coalition. Arsenopyrite (FeAsS), an iron arsenic sulfide, also called mispickel, is the most common mineral from which arsenic is obtained, according to Los Alamos.
Arsenic was known as early as the fourth century B.C., when Aristotle referred to one of its sulfides as "sandarach," or red lead, according to Chemicool. Albertus Magnus, a German philosopher and alchemist, first isolated the element in 1250.
The word arsenic comes from the Persian word "zarnikh," meaning "yellow orpiment," which the Greeks adopted as "arsenikon," according to the Los Alamos National Laboratory. The word is also related to the Greek word "arsenikos," meaning "masculine" or "potent." The Latin word for it became "arsenicum."
Under standard atmospheric pressure, arsenic sublimes, or changes directly from the solid state to the gaseous state without becoming a liquid. However, it will turn into a liquid when put under high pressure.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Arsenic has a number of forms, or allotropes. The most common is metallic gray, followed by yellow and then black. Gray arsenic, the only form used in industry, is the most stable of the three and the strongest conductor of electricity.
Arsenic occurs naturally in the environment in both organic (arsenic atoms bonded with carbon) and inorganic (no carbon) forms. Inorganic arsenic, the most abundant type, occurs with many other elements, particularly sulfur, oxygen and chlorine. Inorganic arsenic is the type associated with more adverse health effects for humans.
Just the facts
- Atomic number (number of protons in the nucleus): 33
- Atomic symbol (on the periodic table of the elements): As
- Atomic weight (average mass of the atom): 74.92160
- Density: 5.776 grams per cubic centimeter
- Phase at room temperature: solid
- Melting point: 1,502.6 degrees Fahrenheit (817 degrees Celsius)
- Boiling point: 1,117.4 F (603 C)
- Number of isotopes (atoms of the same element with a different number of neutrons): 33; 23 whose half-lives are known; 1 stable
- Most common isotopes: As-75 (100 percent natural abundance)
Dangers of arsenic
Even when there is no foul play involved, arsenic still poses a danger, as lethal levels may be leaked into people's water, food or air supply. The most urgent concern is drinking water, and for some places, the risk of arsenic contamination is particularly high.
In 2001, the Environmental Protection Agency (EPA) adopted a lower standard for arsenic in drinking water. The new arsenic standard of 10 parts per billion (ppb) replaced the old standard of 50 ppb.
Bruce A. Stanton, a professor in the department of microbiology and immunology at the Geisel School of Medicine at Dartmouth College in New Hampshire, said that in many states, "arsenic can be found in well water at levels above the EPA standard of 10 ppb."
"Well-water arsenic is above the EPA standard in as many as one in five wells in New Hampshire and many other states, including Maine, Michigan, California, New Mexico, Arizona, Colorado and Nevada," he told Live Science.
As for food, the Food and Drug Administration (FDA) recently turned its attention toward rice, as it tends to absorb arsenic more readily than other crops do. And since rice is a staple in the diets of many infants and young children, the FDA has been closely monitoring rice for safety, ensuring that infant rice cereal stays under 100 parts per billion (ppb) for levels of inorganic arsenic.
One study, published in the Nutrition Journal, suggested that other types of foods, including white wine, beer and Brussels sprouts, may be linked to higher levels of arsenic in humans as well. The FDA has also taken steps to monitor apple juice.
Health problems
Arsenic poisoning can cause all sorts of health problems. A large dose can cause immediate sickness and death, while long-term exposure is associated with higher rates of skin, bladder and lung cancers, as well as heart disease, according to the FDA.
"At levels found in well water in the U.S. (10-100 ppb), ingestion of well water containing arsenic reduces IQ and has many other adverse health effects, include birth defects," said Stanton. "The good news is that arsenic can be detected in well water by inexpensive tests and we can protect ourselves from arsenic exposure in well water by filtration (Zero Water tabletop water filters) and other methods."
An essential nutrient
Paracelsus, a 16th-century Swiss German philosopher and toxicologist, once famously said, "all things are poison, and nothing is without poison. Only the dose permits something not to be poisonous."
But can a toxic element really be necessary for life? A growing body of evidence says yes. Some toxic metals, in trace amounts, might actually be essential nutrients, according an analysis in the journal EMBO Reports. In fact, scientists have found that the body needs arsenic, at a level of 0.00001 percent, to grow and maintain a healthy nervous system, according to Chemicool.
Who knew?
- As early as 82 B.C., the Roman dictator Lucius Cornelius Sulla attempted to end a rash of arsenic poisonings by passing the Lex Cornelia, the first known law against poisoning, according to Dartmouth College.
- In 1836, a British chemist named James Marsh finally developed a test that could detect minuscule amounts of arsenic in both food and human remains. The epidemic of arsenic poisoning finally began to wane.
- Although unproven, there is a persistent rumor that Napoleon Bonaparte was slowly poisoned with arsenic by someone in his cortege, which eventually led to his death in 1821. And while it is generally recognized today that he died of an advanced case of gastric cancer, many still believe that arsenic played a role.
- Perhaps the most famous of arsenic poisoners were the Borgias, a power-grabbing family in Italy who, partly due to their strategic arsenic poisonings of the wealthy and prominent, soon became the most powerful family during the Renaissance period.
- In the Victorian era, white arsenic, or arsenic trioxide (As2O3), was widely available and sold in grocery stores. Women would eat or rub arsenic mixed with vinegar or chalk into their skin as a complexion-enhancer, trying to make their skin paler to show they did not work in the fields.
Agricultural use
Since arsenic is such a strong toxin, farmers, as well as the U.S. government, in the early 20th century believed it would be a good idea to make rodent poisons and crop pesticides out of the substance. It took several decades for everyone to realize what a terrible idea it was to spray this carcinogenic chemical on the food supply. In the 1980s, all of these arsenate pesticides were finally banned, but some of the residue still lingers in the soil today, according to The Lead Group, Inc.
Starting in the 1940s, arsenic-treated wood preservatives, such as chromated copper arsenate (CCA), were widely used to prevent rotting in lumber. Although these preservatives still aren't officially banned, manufacturers voluntarily stopped the production of arsenic-treated wood products in 2003, according to the EPA.
Medical use
In 1786, a British physician named Thomas Fowler presented his arsenic-based, cure-all tonic known as Fowler's solution. The tonic was commonly used to treat skin conditions, such as psoriasis. Unfortunately, it became apparent that people who used the product had a significantly higher risk of developing cancer, particularly on the exact spot the solution was applied. Its use was phased out between the 1930s and 1950s, according to Dartmouth College.
In 1910, German pharmacologist Paul Ehrlich developed the arsenic-based drug Salvarsan, also known as arsphenamine, as a treatment for syphilis, a disease that was endemic and incurable at the time. The drug was incredibly effective and remained the top medicine for curing syphilis until penicillin became available in the 1940s, according to Chemical & Engineering News.
Ehrlich's development of Salvarsan was the first step toward targeted chemotherapy. Today, arsenic trioxide is a very effective drug used to treat people with acute promyelocytic leukemia, according to Stanton.
Industrial use
Arsenic is sometimes alloyed with lead to form a harder, more durable metal. Some areas of use include car batteries and bullets. Until recently, arsenic was commonly used in glassmaking. However, due to pressure from the EPA and environmentalists, most glass manufacturers have slowed down or stopped using arsenic.
According to the Los Alamos National Laboratory:
- Arsenic is often used as a doping agent for solid-state devices such as transistors.
- Gallium arsenide is used in lasers that convert electricity into coherent light.
- Arsenic compounds, such as Paris green, calcium arsenate and lead arsenate, were used as insecticides and in other poisons.
- Arsenic is used in pyrotechnics to give additional color to the flame.
- Arsenic improves the sphericity of lead shot.
Additional resources

 Live Science Plus
Live Science Plus