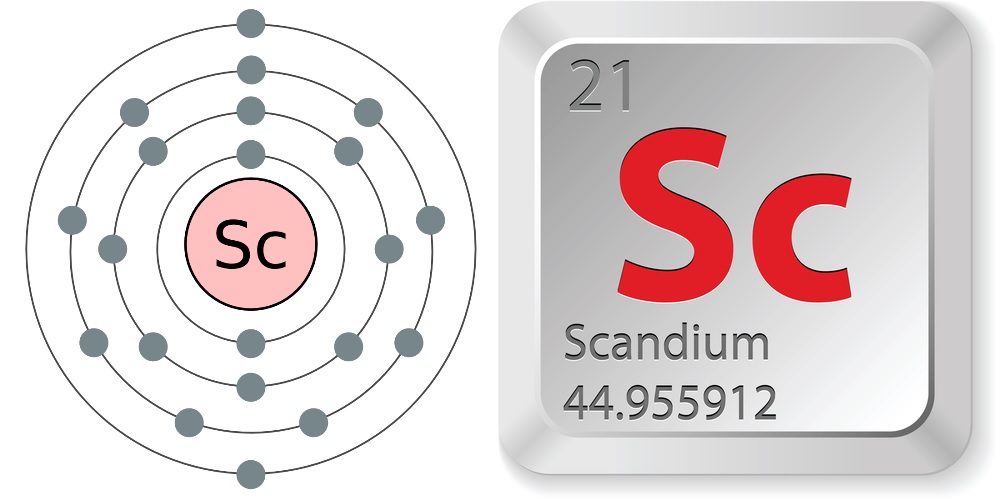
Facts About Scandium

Scandium is a silvery metal that is soft and has a density of about three times that of water. Scandium is a transition metal and is also considered a rare earth element due to similar chemical properties (such as difficulty in extracting and in separating from other elements) and existence in the same ores, according to Chemicool.
Scandium is the 31st most abundant element on Earth, according to Periodic Table, with about 22 parts per million abundance by weight in Earth's crust, according to Chemicool.
Scandium is scattered thinly and has been found in over 800 minerals. Within these minerals, scandium is found in its oxide form (Sc2O3, also known as scandia or scandium oxide), according to Scandium Mining. There are many commercial uses for scandium even though the cost of scandium is typically high – several thousand dollars per kilogram for scandium oxide and up to a few hundred thousand dollars per kilogram for pure scandium, according to Chemistry Explained.
Just the Facts
- Atomic number (number of protons in the nucleus): 21
- Atomic symbol (on the periodic table of elements): Sc
- Atomic weight (average mass of the atom): 44.9559
- Density: 1.734 ounces per cubic inch (3.0 grams per cubic cm)
- Phase at room temperature: solid
- Melting point: 2,804 degrees Fahrenheit (1,540 degrees Celsius)
- Boiling point: 5,126 F (2,830 C)
- Number of natural isotopes (atoms of the same element with a different number of neutrons): 1. There are also 13 artificial isotopes created in a lab.
- Most common isotopes: Sc-45 (100 percent of natural abundance)
History
Russian chemist Dmitri Mendeleev, inventor of the periodic table, predicted the existence and properties of scandium (which he called "ekaboron" — similar to boron) in 1869, according to the New World Encyclopedia. Lars Fredrick Nilson, a Swedish chemist, when examining the spectra of the minerals euxenite and gadolinite, discovered the element in 1879. The element was named for the Latin word for Scandinavia, "Scandia," due to the minerals in which scandium was at that time found to exist only in the Scandinavian Peninsula, according to Peter van der Krogt, a Dutch historian. Per Teodor Cleve, a Swedish chemist, made the link that the new element discovered by Nilson was the same as the proposed element described by Mendeleev.
In the first attempt to isolate scandium, Nilson and his team processed 10 kilograms of euxenite and were able to produce about two grams of scandium oxide. According to the New World Encyclopedia, metallic scandium wasn't produced for the first time until 1937 by Werner Fischer, Karl Brünger, and Hans Grienseisen, German chemists. In 1960, 99 percent pure scandium was produced.
Who knew?
- Scandium oxidizes when exposed to air and its color will be tinged with yellow or pink due to the formation of scandium oxide on the surface, according to the Encyclopedia Britannica.
- Scandium is more abundant on the moon (according to ScandiumFacts) and the sun (according to Los Alamos National Laboratory) than on Earth. And on Earth, the scandium tends to be spread thinly across the surface rather than concentrated in any certain locations.
- According to Minerals Education Coalition, scandium is mainly mined in China, Kazakhstan, Madagascar, Norway and Russia with a good deal coming from Cold War-era stockpiles in the former Soviet Union (according to New World Encyclopedia).
- According to Encyclopedia, the total amount of scandium produced in the United States is regarded as a "trade secret in the industry" and is not announced.
- According to Lenntech, scandium plays no roles in biology, and the average person ingests less than 0.1 microgram per day with no known health effects.
- According to Scandium.org, scandium-aluminum alloys have improved strength, improved resistance to recrystallization, reduced grain size, and improved resistance to hot cracking over conventional alloys with even as little as 0.5 percent of the alloy being scandium.
- Scandium is mainly used to create high-intensity lighting and mercury vapor lamps (used mainly for photography and television/movies) Scandium produces light that is very similar to natural sunlight, according to Chemicool. According to David Darling, a British astronomer and writer, scandium is also used in alkaline batteries, as a catalyst (as scandium oxide), and to improve germination of plant seeds (as scandium sulfate).
- The U.S. Geological Survey reports that the highest uses of scandium in 2015 were in solid oxide fuel cells and aluminum-scandium alloys with smaller amounts of scandium used in ceramics, electronics, lasers, and radioactive isotopes.
- Scandium is also used in alloys to make specialty sporting equipment, including bicycle frames and baseball bats, according to Chemistry Explained. The benefits of using scandium alloys versus conventional alloys for sporting equipment are increased strength, lighter weight, and a resistance to corrosion.
- A 2003 study by Zaki Ahmad, an engineer from Saudi Arabia, published in the Journal of the Minerals, Metals, and Materials Society described some applications of scandium-reinforced aluminum, including those described above as well as aerospace applications (bulk heads, heat shields, wheels, fuel and exhaust systems, and more), automotive and air transportations (wheels, bumpers, frames, pistons, air bag canisters), and be used in saltwater environments such as in desalination plants and in boat engines.
- According to Ahmad, , the first uses for scandium-aluminum alloys were primarily fueled by the Soviet military. The alloys were mainly used on the MIG 29 fighters and in missiles due to their advantages over the most common alloys at the time (mainly aluminum-magnesium and aluminum-lithium).
- According to the Royal Society of Chemistry, radioactive isotope scandium-46 is used in oil refining as a tracer to monitor the oil movement and in underground pipes to detect leaks.
- According to Minerals Education Coalition, hydrogen gas is released during a reaction when water runs over scandium due to a reaction with the oxygen in the water.
- According to New World Encyclopedia, scandium impurities give the blue color to the variety of beryl known as the aquamarine gemstone.
Current research
The majority of research involving scandium is the use of scandium alloys, particularly with aluminum. Most of these studies discuss the benefits of combining scandium with other metals and what alloys will possibly be used for. There are new methods in working with the alloys, such as a patent filed in 1997 by William Tack, an American inventor, and continuing studies on recovering scandium from various resources, such as this study by Weiwei Wang et al., a group of Australian researchers, published in 2010 in Elsevier in the Hydrometallurgy section.
Tack's patent described a method for using aluminum-scandium alloys to assemble structures such as bicycle frames by use of welding. The alloys that were used contained various amounts of scandium along with other "fillers" including silicon, manganese, magnesium, zirconium, titanium, and copper to determine the best combination of materials for various structures using the author's welding technique.
Get the world’s most fascinating discoveries delivered straight to your inbox.
The 2010 study described several processes for the recovery of scandium from several resources. Scandium is primarily recovered as a by-product of mining other ores such as uranium, aluminum, titanium, zirconium, and other rare-earth ores. Various techniques are used to recover the scandium in the left over ores including leaching, solvent extraction, precipitation, and calcination.
A dissertation study published in 2016 by Claire Parker, an ocean sciences student in California, discusses the importance of scandium in the ocean. Trace metals are essential micronutrients in the health of the ocean environment, and while iron is the most studied, scandium has been one of the least studied trace elements that are found in the ocean. There are no known biological roles for scandium. Therefore, according to the author, the scandium dissolved in the ocean could relate to the non-nutrient side of the oceanic iron cycle.
Based on several studies and discussed in the dissertation, dissolved scandium and iron in the ocean have similar distributions, concentrations and reactivity (based on a similar inorganic compound). The author concludes that studying both iron and scandium and their properties within the ocean can provide "new insights into physical, chemical, and biological processes occurring in the ocean."
Additional resources

Rachel Ross is a science writer and editor focusing on astronomy, Earth science, physical science and math. She holds a Bachelor of Arts in Philosophy from the University of California Davis and a Master's degree in astronomy from James Cook University. She also has a certificate in science writing from Stanford University. Prior to becoming a science writer, Rachel worked at the Las Cumbres Observatory in California, where she specialized in education and outreach, supplemented with science research and telescope operations. While studying for her undergraduate degree, Rachel also taught an introduction to astronomy lab and worked with a research astronomer.