Facts About Magnesium

Magnesium makes up 2 percent of the Earth's crust, but you won't see this silvery, light metal in nature. This versatile element occurs naturally only in combination with other elements, such as carbon, calcium and oxygen, as in the common mineral dolomite.
Magnesium wasn't purified until 1808, when Cornish chemist Sir Humphry Davy, who created a small amount by running an electrical current through magnesium oxide, according to the Royal Society of Chemistry. In 1831, French chemist Antoine Bussy was the first to create a significant amount of pure magnesium, according to the RSC.
The metal can be mixed with other metals, particularly aluminum, for use in making car bodies, drink cans and other items that need to be light and strong. Magnesium is flammable, so one of its main uses is for flares and fireworks. During World War II, the element was even used to make incendiary bombs.
Just the facts
According to the Jefferson National Linear Accelerator Laboratory, the properties of magnesium are:
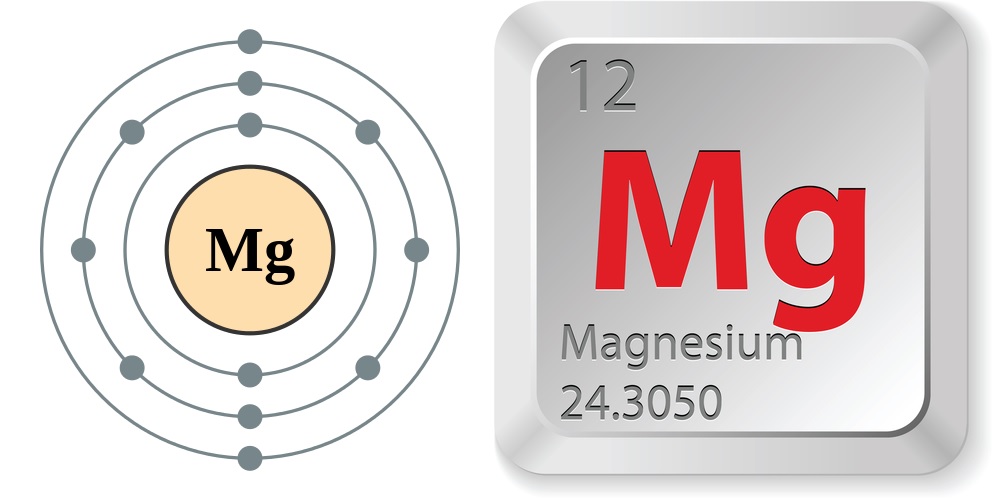
- Atomic number (number of protons in the nucleus): 12
- Atomic symbol (on the Periodic Table of Elements): Mg
- Atomic weight (average mass of the atom): 24.3050
- Density: 1.74 grams per cubic centimeter
- Phase at room temperature: Solid
- Melting point: 1,202 degrees Fahrenheit (650 degrees Celsius)
- Boiling point: 1,994 F (1,090 C)
- Number of isotopes (atoms of the same element with a different number of neutrons): 21; 3 stable
- Most common isotopes: Mg-24 (78.99 percent natural abundance), Mg-25 (10 percent natural abundance), Mg-26 (11 percent natural abundance)
Biological metal
Magnesium is born in stars, when, under extreme temperatures, helium and neon fuse. It's the eighth-most abundant element in the universe, according to the U.S. Geological Survey. On Earth, magnesium is found both in the crust and in the mantle; it's also the third-most abundant mineral dissolved in seawater, with a 0.13 percent concentration.
After Davy isolated magnesium in 1808, several scientists worked to purify the metal using various methods, according to the USGS. It wasn't until 1909, though, that an electrolysis method, developed by Robert Bunsen (inventor of the Bunsen burner), made industrial production possible.
Magnesium burns too easily to be used widely for building, according to the Jefferson Lab, but when mixed with aluminum, it creates an alloy that is strong, light and easy to work with.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Magnesium also has biological uses. It's part of chlorophyll, the green pigment that plants use to extract energy from sunlight. The element is also crucial to more than 300 biological processes in the human body, according to the U.S. National Library of Medicine. Adult women should take in about 320 milligrams of magnesium a day, and adult men 420 milligrams, according to the NLM. Fruits, vegetables, nuts, legumes and whole grains are good sources. Doctors sometime recommend magnesium supplements for a variety of medical conditions, including high blood pressure, premenstrual syndrome and diabetes.
Who knew?
- The word "magnesium" comes from the name of the Greek region Magnesia, where compounds of this element occur naturally.
- Milk of Magnesia, which works as a laxative and to treat indigestion, is a compound of magnesium, hydrogen and oxygen molecules.
- Another home remedy owed to magnesium? Epsom salts, otherwise known as magnesium sulfate. The name "Epsom" comes from a spring in England where the salts occur naturally.
- The United States holds an estimated 65 metric tons of magnesium in natural magnesite deposits, according to the USGS. Of that, 88 percent is found in Nevada.
- Don't put out a magnesium fire with water: H20 only makes a magnesium metal fire flare up. Car and appliance manufacturers are increasingly using magnesium in their products, according to Firehouse, an online publication for firefighting news. For firefighters, that means the need for additional training and safety procedures.
Current research
Imagine a bandage that would kill bacteria from inside a wound. That's the goal of Tufts University biomedical engineer Fiorenzo Omenetto and his colleagues, who have created an implantable device of silk and magnesium that can knock out a staph infection before dissolving harmlessly inside the body.
Typically, medical implants are made of metal or other permanent materials. That means they either become part of the body forever, or they must be removed, a process that can carry risk of infection or other complications.
Omenetto and his team are working to develop something very different. Using a substrate of silk — which is made up of amino acids, the building blocks of protein — and a coil of magnesium, the researchers were able to create a simple miniature heater. Once implanted into the body, the heater can be turned on, increasing the temperature of the tissue just enough to kill Staphylococcus aureus bacteria. After days or weeks, depending on the construction of the silk and the thickness of the magnesium, the device dissolves. Tests in mice found that the device successfully knocked out the bacteria, and that in the aftermath, the tissue was perfectly healthy, with no signs of increased levels of silk proteins or magnesium. The researchers reported their findings in November in the journal Proceedings of the National Academy of Sciences.
Magnesium made a good candidate for the coil, Omenetto told Live Science, because it breaks down in contact with body fluids and because it is so biocompatible. The recommended daily allowance for magnesium in the diet, according to the Institute of Medicine, is between 300 and 400 milligrams per day. The heater implant contains a mere 30 micrograms of magnesium, a drop in the bucket in comparison.
"When you examine the area around the tissue, you don't find traces above what you would find physiologically," Omenetto said. "these are encouraging signs that these devices just kind of go away."
The device is far from hitting the medical market, and the research is very much in the "discovery-driven" stage, he said. In theory, however, biodegradable silk-and-magnesium devices could also be used for other purposes, like sensors for food to detect spoilage.
Follow Live Science @livescience, Facebook & Google+.
Additional resources

Stephanie Pappas is a contributing writer for Live Science, covering topics ranging from geoscience to archaeology to the human brain and behavior. She was previously a senior writer for Live Science but is now a freelancer based in Denver, Colorado, and regularly contributes to Scientific American and The Monitor, the monthly magazine of the American Psychological Association. Stephanie received a bachelor's degree in psychology from the University of South Carolina and a graduate certificate in science communication from the University of California, Santa Cruz.