Science Fiction Turned Fact: Water Powered Cars
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
This Research in Action article was provided to LiveScience in partnership with the National Science Foundation.
One of engineering’s primary challenges lies in solving the world’s energy crisis. A major part of the energy solution lies in eliminating fossil fuels as transportation fuels. The idea of a car running on sun and water would have been viewed as science fiction as recently as 10 years ago. However, several automotive manufacturers are realizing that vision, through development of fuel cell powered vehicles driven by hydrogen generated from water and solar energy. The automotive industry is resolute that it will be commercializing those fuel cell cars in the next two to three years. A key enabler in that transition will be the fueling stations to support the cars.
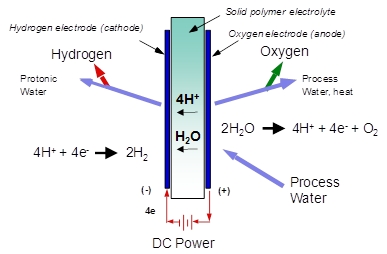
Proton OnSite, which designs and manufactures hydrogen and gas systems, is playing a key role in proliferating those stations with its sister company, SunHydro. Its systems incorporate renewable energy and water to produce a fuel of tomorrow. They use electricity to split hydrogen from water, in fuel cells incorporating catalysts that reduce the energy required, as well as membranes to separate the hydrogen from oxygen and water. The technology is enabled by the membrane’s ability to separate out positive ions.
That membrane acts as both the conductive bridge between the two electrodes for the electrochemical reaction, and the barrier that separates the hydrogen from the oxygen. In conjunction with Proton’s cell design, membrane thicknesses on the order of the diameter of a human hair can separate hydrogen at pressures of over 2,400 pounds per square inch from oxygen at ambient pressure. The traditional process for fabricating synthetic membranes is expensive, and most membranes do not maintain robustness at those high pressures.
Through a National Science Foundation-funded program, Proton OnSite has been developing and testing new membrane materials for increased mechanical strength and higher operational efficiency. The new materials are aimed at replacing current state-of-the-art membranes, with cost, durability and efficiency improvements that would allow their use in energy and fueling markets. The proposed materials, if successful, would represent a 75 percent cost savings over current membranes and enable higher temperature operation for improved efficiency. The result would be deployment of commercial technology that could help revolutionize the way we fuel our cars and reduce our carbon footprint.
Editor's Note: Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author and do not necessarily reflect the views of the National Science Foundation. See the Research in Action archive.
Get the world’s most fascinating discoveries delivered straight to your inbox.
 Live Science Plus
Live Science Plus