Unwinding While Staying on Track: That's What the Body's Helicases Do
Like "The Little Engine That Could," helicases are hardworking enzymes that don't give up. Without them, your cells would stop dividing and many other important biological processes would come to a halt.
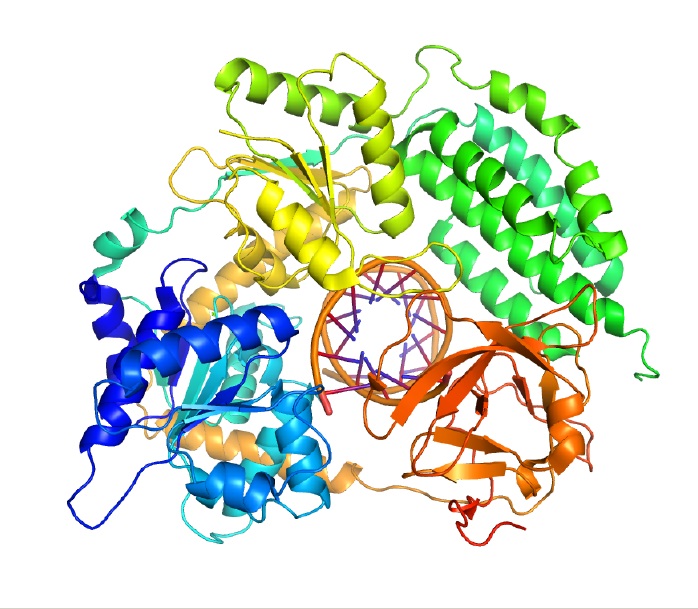
Helicases are involved in virtually all cellular processes that involve DNA and RNA. Their claim to fame, though, is unwinding DNA so it can be copied during cell division. Helicases are evolutionarily ancient enzymes that are found in viruses and in all living things. Most organisms — including humans — have many versions, attesting to these enzymes' critical and diverse roles inside cells. The human genome encodes 95 helicase forms. Even the microscopic E. coli bacterium has more than a dozen helicases.
When something goes wrong with helicases, it can cause health problems. Mutations that disable helicases have been linked to cancer and certain genetic diseases, such as Werner syndrome (a premature aging condition) and xerodermapigmentosum (a photosensitivity disorder caused by a defect in DNA repair).
Read on to find out some of the latest discoveries made by scientists funded by the National Institutes of Health about how helicases keep us alive and well.
Staying on Track
Before a cell divides, it needs to copy its DNA so that each "daughter" cell gets a complete set of chromosomes. Helicases unwind and separate the DNA strands to make way for the duplication machinery.
Helicases chug along vast stretches of DNA without falling off, keeping pace with the DNA replication machinery that follows behind. With many organisms having millions, if not billions of nucleotide "letters" in their DNA, helicases also must work quickly to help duplicate it all. Studies have shown that helicases can travel at breakneck speeds, barreling past hundreds of nucleotides per second.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Even when jetting along DNA at top velocities like the Shinkansen, helicases have a remarkable ability to hang onto the DNA strand without falling off. Researchers have wondered how helicases stay on track for so long when some other enzymes have trouble sticking. Michelle Wang, a physicist at Cornell University, and Smita Patel, a biochemist at the Robert Wood Johnson Medical School at the University of Medicine and Dentistry of New Jersey, recently helped shed light on this question.
Like many other helicases, the one they studied is made up of six protein parts arranged in a ring. The DNA strand passes through the center of the ring. The researchers discovered that two of the helicase protein parts move along the strand while the other four tether it to the DNA, allowing the helicase to advance while staying securely on track.
Helicase Superheroes
While staying firmly tethered, certain helicases can also knock off unwanted proteins that stand in their way. This unexpected role turned up in recent research led by physicist Taekjip Ha of the University of Illinois.
During the copying process, unzipped DNA is exposed to potential hijacking by proteins that could shuffle around the genetic material in harmful ways. Ha's team discovered that a helicase called PcrA safeguards against hijacking by repeatedly reeling in and releasing exposed DNA strands, knocking off any unwelcome proteins that could damage them.
Sensing RNA Viruses
Many scientists are working to uncover the roles played by RNA helicases, which are less understood than their DNA counterparts. While many RNA helicases are involved in producing, processing or using RNA, others play an unusual role by helping to fight viral infection.
When an RNA virus invades a cell, it produces RNA molecules that help to propagate the virus and thus the infection. An RNA helicase called RIG-I helps check the infection by recognizing the viral RNA molecules and calling in the innate immune system— the body's first line of defense against invading pathogens.
Smita Patel, this time in collaboration with structural biologist Joseph Marcotrigiano of Rutgers University, produced detailed pictures of how RIG-I binds to viral RNA. The team's molecular snapshots showed that binding to the RNA substantially shifts RIG-I's structure and unmasks a region that sounds the immune system alarm.
Scientists can use this new knowledge as they seek to design drugs that act on RIG-I to fight infections or control an overactive immune response.
This Inside Life Science article was provided to LiveScience in cooperation with the National Institute of General Medical Sciences, part of the National Institutes of Health.
Read more: