Ozempic-like meds linked to higher risk of pancreatitis, 'stomach paralysis' than other weight-loss drugs
GLP-1 agonists like Ozempic come with a higher risk of severe GI issues than a different common weight-loss drug does.
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
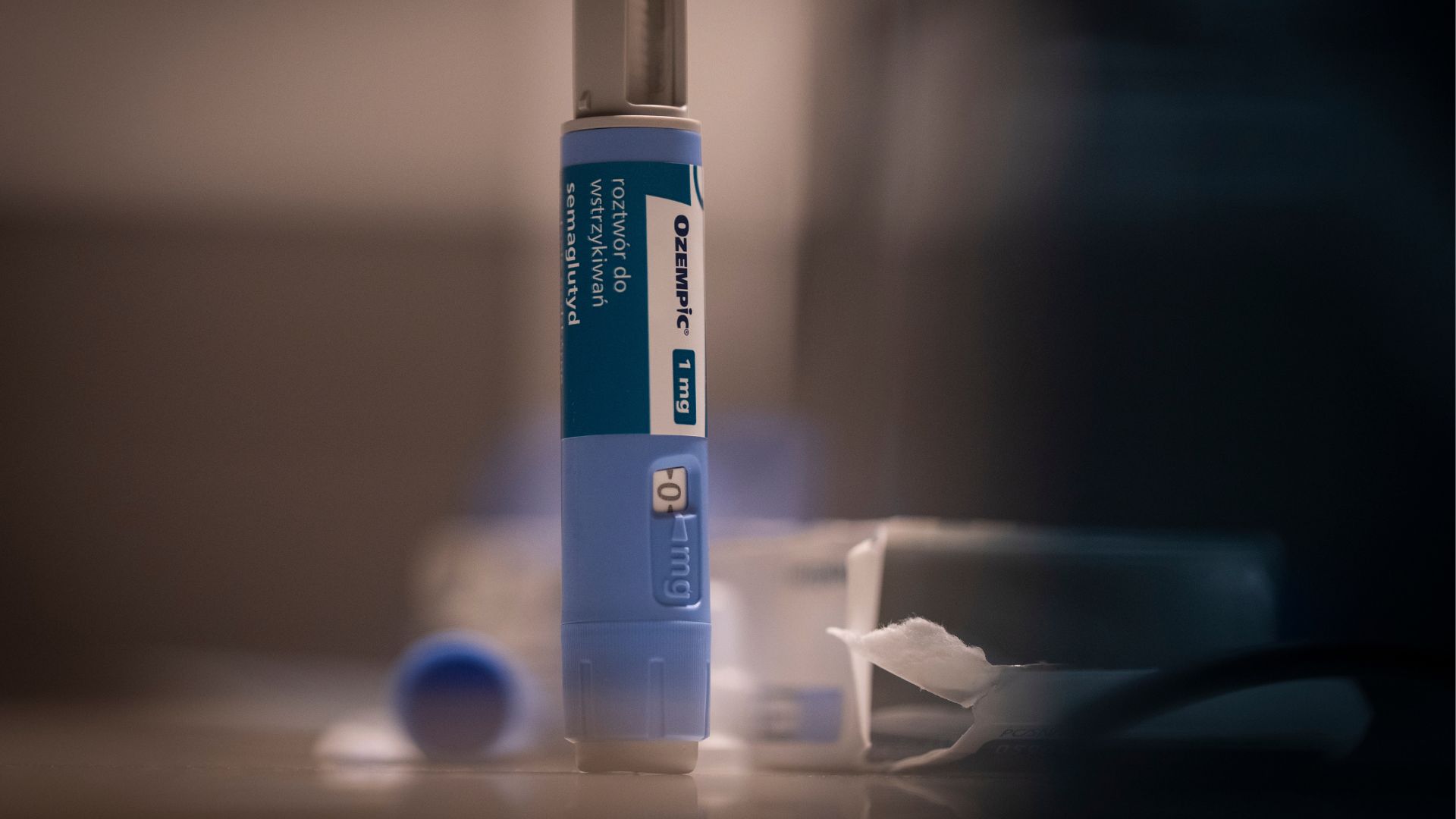
Taking drugs like Ozempic for weight loss may come with a slight risk of severe gastrointestinal (GI) problems, including inflammation of the pancreas and too-slow stomach movements, sometimes called "stomach paralysis."
That's according to a new study published Thursday (Oct. 5) in the journal JAMA. In the work, scientists compared the rate of serious GI problems in people who took Ozempic-like drugs, called GLP-1 agonists, for weight loss with the rate seen in people who took a different type of weight-loss drug called bupropion-naltrexone.
Those who took GLP-1 agonists had higher rates of bowel obstruction, pancreatitis and gastroparesis. Also called "stomach paralysis" or "delayed gastric emptying," gastroparesis refers to the slowing or stopping of stomach contractions, which causes food to linger too long and potentially form masses called "bezoars."
"Given the wide use of these drugs, these adverse events, although rare, must be considered by patients thinking about using them for weight loss," study first author Mohit Sodhi, a medical student at the University of British Columbia, said in a statement.
Related: Can Ozempic and Wegovy cause 'stomach paralysis' and 'cyclic vomiting'?
That's especially true for people who do not have diabetes, because the drugs were originally approved to treat type 2 diabetes. Thus, the most robust research into their side effects comes from people with the condition. However, the drugs have also been prescribed "off-label" for weight loss, and since 2021, there are versions of the drugs specifically marketed for weight loss. (Ozempic, for example, is approved for diabetes, but the same drug is now available under the name Wegovy as a weight-loss treatment.)
Recent trials that studied GLP-1 agonists for weight loss were limited in their size and length, which left a gap in our understanding of the drugs' side effects, the authors wrote in their paper. People with diabetes have an elevated risk of severe GI issues at baseline, so the risks for people without diabetes may differ, they noted.
Get the world’s most fascinating discoveries delivered straight to your inbox.
"People who are otherwise healthy may be less willing to accept these potentially serious adverse events," Sodhi said.
The new study used PharMetrics Plus, a massive database of health insurance claims filed in the U.S. In the data, the team identified people who took the GLP-1 agonists semaglutide and liraglutide; semaglutide is in Ozempic and Wegovy, while liraglutide is in Saxenda. All of these drugs work by mimicking a hormone that helps control appetite and the speed at which the stomach empties.
The team compared GLP-1 users with those taking bupropion-naltrexone, a different weight-loss drug that works by tweaking signals in the brain's reward center that would usually spur a person's appetite. About 650 people in the study were taking bupropion-naltrexone, 600 were taking semaglutide and 4,100 were taking liraglutide. All of the participants had obesity but not diabetes.
Compared with bupropion-naltrexone users, GLP-1 users had a roughly ninefold greater risk of pancreatitis, a fourfold higher risk of bowel obstruction, and a 3.6 times higher risk of gastroparesis.
The overall rate of these conditions was still fairly low, though. For example, out of 1,000 people taking semaglutide for one year, there were about five cases of pancreatitis. Among liraglutide users, there were closer to eight cases. Gastroparesis affected about 10 in 1,000 semaglutide users and 7 in 1,000 liraglutide users in a year. Bowel obstruction affected 8 in 1,000 liraglutide users, but there were no cases among semaglutide users.
Doctors told NBC that they wonder whether some of these side effects were linked to the patients' rapid weight loss, rather than to the specific drugs used. Others said the study was unlikely to change how they prescribe GLP-1 agonists, but it does emphasize that patients should be closely monitored by their health care providers while taking the drugs.
This article is for informational purposes only and is not meant to offer medical advice.

Nicoletta Lanese is the health channel editor at Live Science and was previously a news editor and staff writer at the site. She holds a graduate certificate in science communication from UC Santa Cruz and degrees in neuroscience and dance from the University of Florida. Her work has appeared in The Scientist, Science News, the Mercury News, Mongabay and Stanford Medicine Magazine, among other outlets. Based in NYC, she also remains heavily involved in dance and performs in local choreographers' work.
 Live Science Plus
Live Science Plus










