New ultrasound device helps powerful chemo reach deadly brain cancers, human trial shows
An implanted ultrasound-emitting device helped chemotherapy drugs safely pass into the brains of cancer patients.
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
The human brain is like a walled fortress: Nutrients, hormones and fuel are allowed to pass through its guarded gates, but pathogens and toxins are locked out. However, this barrier also blocks many drugs from reaching the brain, including potent chemotherapies that could help clear deadly cancers from the organ.
Now, scientists have shown that a new ultrasound device can temporarily open this "blood-brain barrier" in human cancer patients, allowing powerful chemotherapy to reach brain tumors.
The results of the early-stage trial, published Tuesday (May 2) in the journal The Lancet Oncology, provide the first direct evidence that ultrasound can significantly boost the amount of chemo that crosses the blood-brain barrier, the wall of tightly packed cells that lines blood vessels in the organ.
The researchers demonstrated this effect with paclitaxel and carboplatin, two chemo drugs that normally cross the blood-brain barrier in only negligible amounts. Compared with untreated brain tissue, the regions of the brain exposed to ultrasound allowed in about 3.7 times more paclitaxel and 5.9 times more carboplatin, meaning the drugs reached clinically relevant levels.
And then, within about an hour of being "opened," the blood-brain barrier mostly closed back up, the team found, meaning its protective properties had been restored.
"In many ways, this is a critical step," said Dr. Nir Lipsman, a neurosurgeon and director of the Harquail Centre for Neuromodulation at the Sunnybrook Research Institute in Toronto, who was not involved in the trial. The researchers showed in a "systematic and elegant way" that ultrasound can be repeatedly and safely used to deliver chemo into the brain, and that the blood-brain barrier reliably sealed back up after treatment, Lipsman told Live Science.
Related: Brain cancer's 'immortality switch' turned off with CRISPR
Get the world’s most fascinating discoveries delivered straight to your inbox.
Lipsman and others at Sunnybrook also study how ultrasound can be used to usher drugs across the blood-brain barrier to treat diseases such as cancer, Alzheimer's and Parkinson's. They've shown indirectly, through brain scans, that the approach can increase drug concentrations in the human brain. But in the new trial, the team directly measured chemo concentrations in samples of brain tissue, which is considered "gold-standard" evidence, Lipsman explained.
The new trial included 17 adults with recurrent glioblastoma, an aggressive cancer that arises from star-shaped brain cells called astrocytes. The fast-growing tumors spread easily, worming their way through healthy brain tissue in a way that makes them nearly impossible to remove completely through surgery.
After surgery, doctors target any lingering cancer cells with radiation and temozolomide, a fairly weak chemo drug that can cross the blood-brain barrier. These treatments can extend patients' lives, but invariably, glioblastoma is a cancer that "recurs and leads to death in basically all patients that have this diagnosis," Lipsman said. Glioblastoma patients survive an average 15 to 18 months after diagnosis.
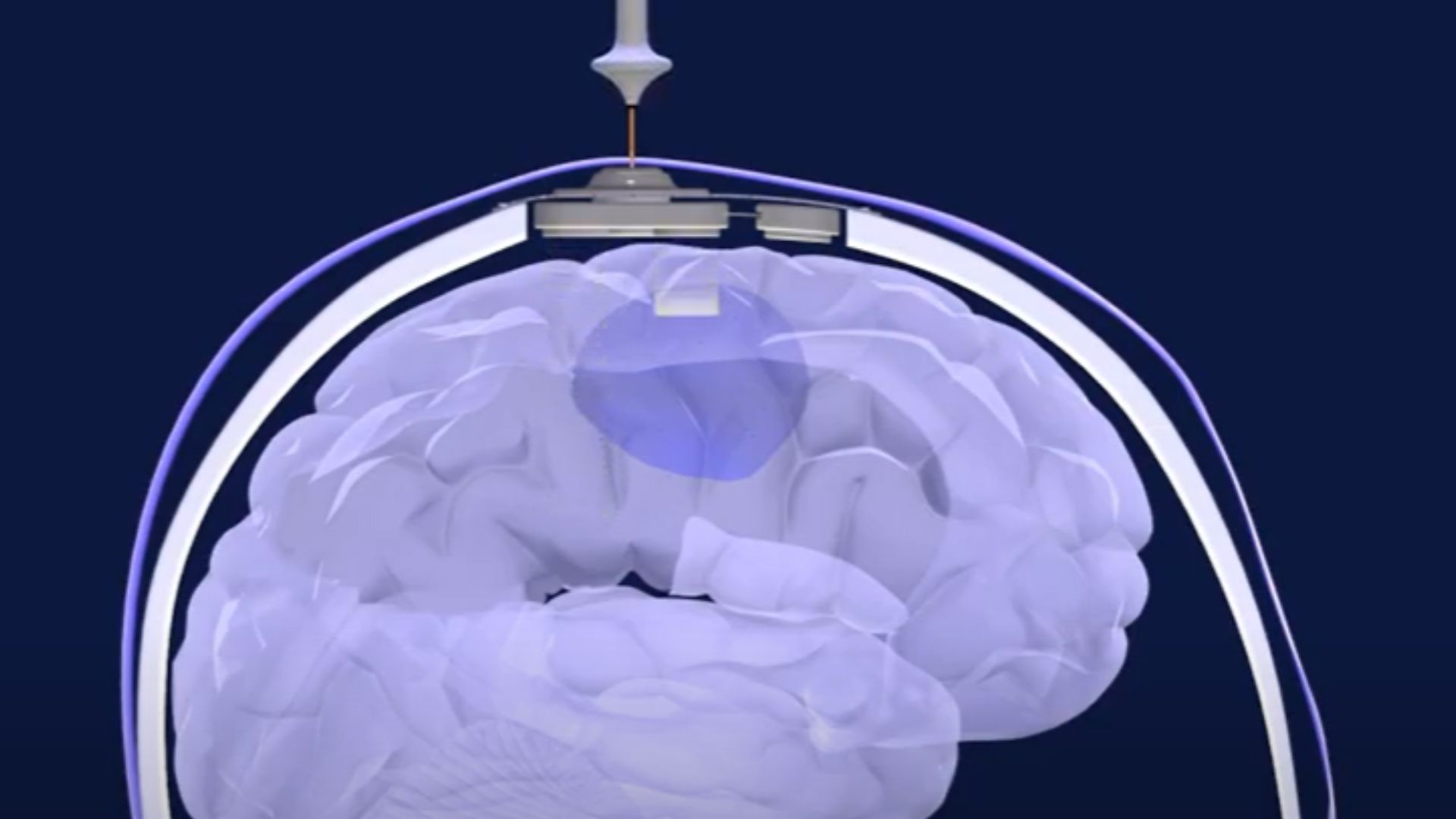
The goal of the new trial was to see if an ultrasound device, implanted in the skull, could help deliver the more-potent chemo drugs paclitaxel and carboplatin into the brain. The team installed the implant, designed by the biotech company Carthera, during each patient's initial surgery to remove as much glioblastoma from the brain as possible.
To use the device, doctors inject microbubbles — tiny spheres of fat filled with gas — into a patient's bloodstream. These microbubbles make their way into the blood vessels of the brain. Upon activation, the ultrasound implant emits sound waves that shake the microbubbles near the device, which, in turn, disrupt the integrity of the blood-brain barrier in the nearby brain tissue.
After less than five minutes of ultrasound pulses, the team administered either paclitaxel or carboplatin. The trial participants received this ultrasound-assisted chemo up to six times, with a three-week gap between each session.
If shorter gaps were taken between sessions, it's possible that harmful side effects, like inflammation, cell death or neurological effects, might have emerged, said Kullervo Hynynen, vice president of research and innovation at Sunnybrook, who was not involved in the trial. But it's unclear how frequent the treatments would have to be to cause such effects, he added.
The treatment schedule used in the current trial appeared safe, and encouragingly, the chemo concentrations delivered into the brain didn't cause serious side effects, Hynynen said.
Related: The 10 deadliest cancers, and why there's no cure
Some of the trial participants had brain tissue near their primary tumors removed, and this gave the researchers the opportunity to sample both ultrasound-exposed and unexposed brain tissue and directly measure how much chemotherapy entered each.
They measured how long the blood-brain barrier stayed open by taking brain scans of the participants before and after treatment. The scans showed that the barrier begins healing very quickly after ultrasound exposure.
"It is consistent with animal data," Hynynen said. "For large molecules, the blood-brain barrier starts healing right away." Prior evidence suggests that the barrier is "practically completely healed" by around six hours post-ultrasound, he added, although some studies have suggested that it sometimes stays open closer to 12 hours, Lipsman said. (This timing may also vary by the region of the brain being targeted, and by the dose of microbubbles and ultrasound used, Lipsman noted.)
The current trial demonstrated that the new ultrasound device is safe and can get chemo into the brain, "but there are really important questions we did not answer," said Dr. Adam Sonabend, an associate professor of neurological surgery at the Northwestern University Feinberg School of Medicine in Chicago and a leader of the trial. For instance, more research is needed to determine the drug combinations, dosing and schedule that are most effective for this treatment method.
One of the biggest questions still to be answered is, "Does this actually translate into making people live longer?" Sonabend said. "This is a question that's obviously very important." On that front, Sonabend and his colleagues are currently recruiting for a larger clinical trial designed to measure how effective this new treatment approach is at killing cancer and prolonging survival.

Nicoletta Lanese is the health channel editor at Live Science and was previously a news editor and staff writer at the site. She holds a graduate certificate in science communication from UC Santa Cruz and degrees in neuroscience and dance from the University of Florida. Her work has appeared in The Scientist, Science News, the Mercury News, Mongabay and Stanford Medicine Magazine, among other outlets. Based in NYC, she also remains heavily involved in dance and performs in local choreographers' work.
 Live Science Plus
Live Science Plus










