Body parts grown in the lab
In recent years, scientists have successfully grown a range of miniature organs and human body parts in the lab.
Regrowing a missing limb is no big deal for creatures that are well-known for their regenerative "superpowers" like starfish or salamanders.
But what about humans?
Individual cells in your body are constantly being replaced as they wear out. For example, the outer layer of your skin is shed roughly every four weeks.
However, regenerating complete organs and body parts is beyond the scope of human biology. Nevertheless, in recent years, scientists have successfully grown a range of replica human body parts in the lab. These include miniature organs, or organoids, that are grown from stem cells, and organ-on-a-chip models, in which cells from specific tissues are grown on credit-card-sized devices that mimic the physiological conditions of an organ in the body.
These approaches allow scientists to study human organs during health and disease at a level of accuracy that was impossible with animal models. As such, it is hoped that they will help expedite the development of new drugs.
Here are some recent examples.
Fallopian tubes
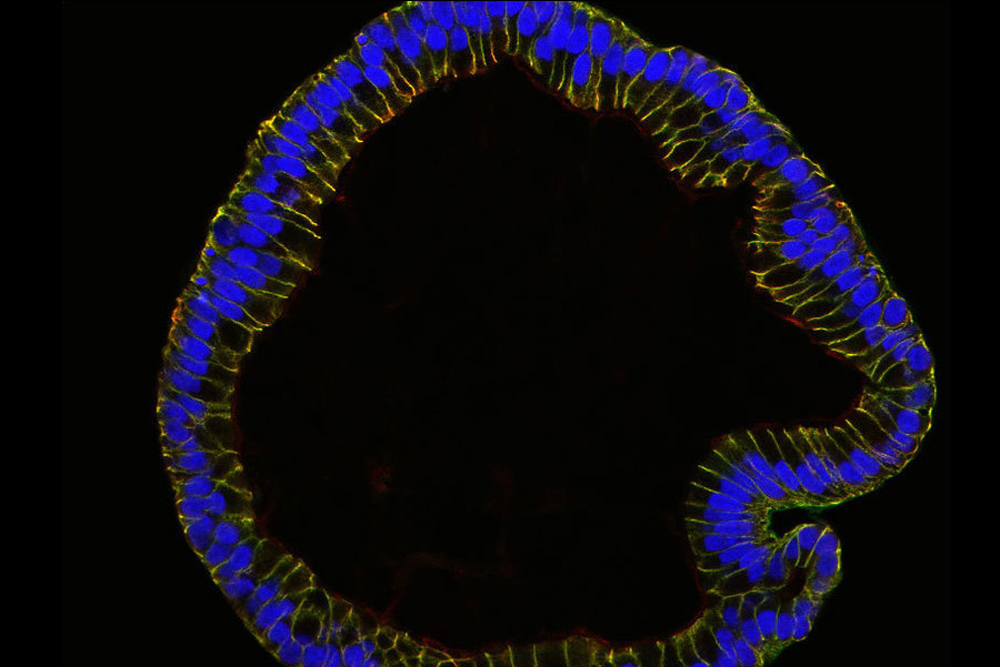
In 2015, scientists used stem cells in a lab dish to grow the innermost cellular layer of human fallopian tubes, the structures that connect the ovaries and the uterus. In an accompanying statement, researchers described the resulting organoids as sharing the features of real, full-size fallopian tubes, such as their characteristic shape. They were even able to identify two signaling pathways that are needed for continuous growth of the organoids, meaning that scientists can study them for longer.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Brain
Over the past decade, researchers have developed increasingly sophisticated miniature, 3D versions of the human brain, or "minibrains", in the lab. Scientists have grown mini models of the human embryonic brain and spinal cord, minibrains that have their own sets of eyes and models specifically grown from fetal brain tissue. Brain organoids are even being grown in space to study accelerated aging and the development of neurological diseases such as Alzheimer's disease.
Heart
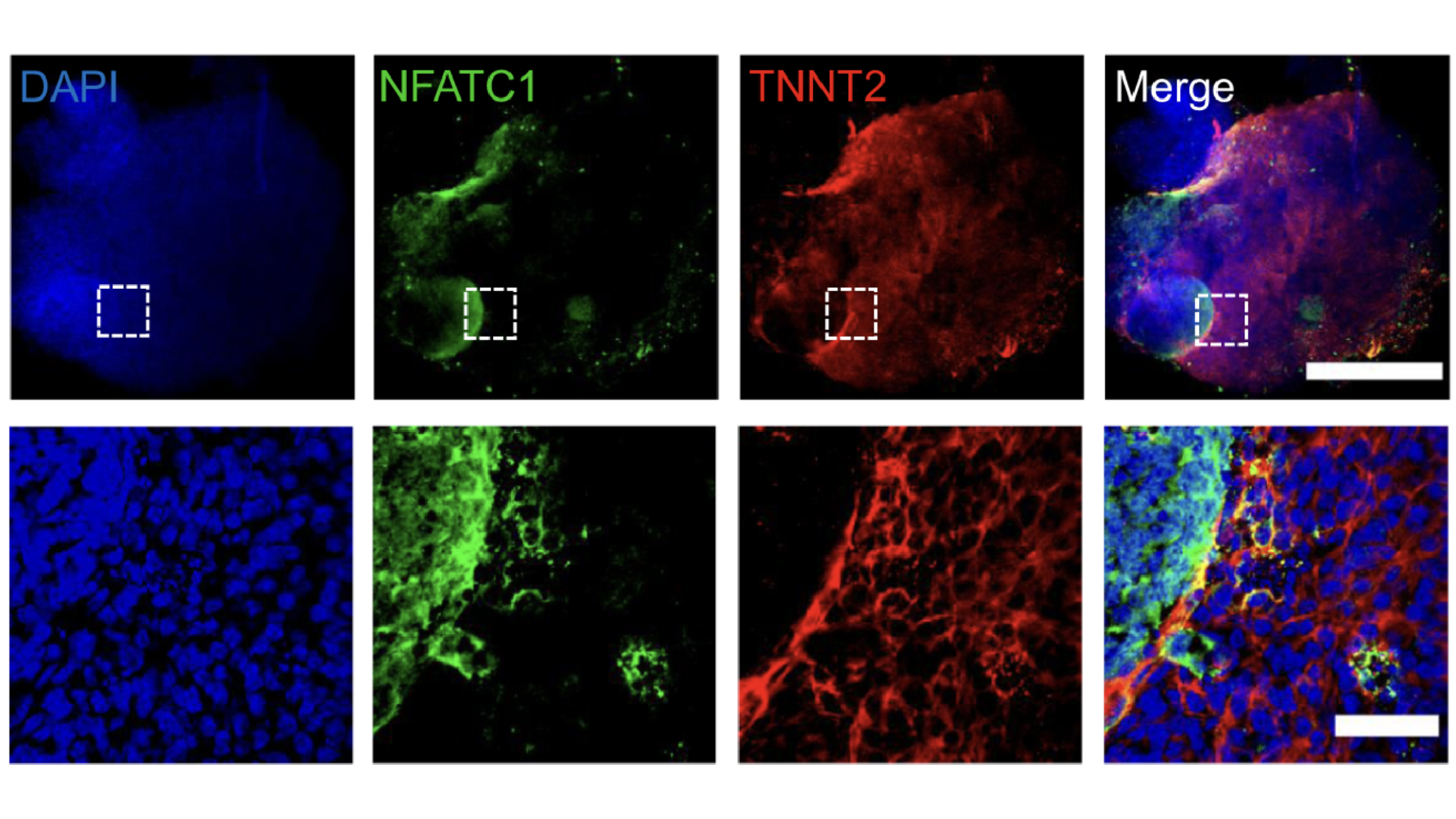
In 2024, scientists used stem cells to grow mini human hearts in the lab. These replica hearts, which are smaller than a grain of rice, have blood vessels and all the cell types that are usually found in the human heart. They even beat like the real thing.
Separately, researchers are also developing so-called heart-on-a-chip devices to study heart disease, as well as how the heart heals after injury, and to evaluate the safety and efficacy of new drugs. Such models have also been sent to space to decipher how microgravity affects the heart.
Kidney
In 2015, scientists grew a mini kidney in the lab. The organoid, which contained all the different cell types found in the human kidney, was grown from stem cells using a combination of growth-inducing chemicals. It resembled the kidney of a developing fetus and could be used for drug testing, said the team who developed it in an associated statement.
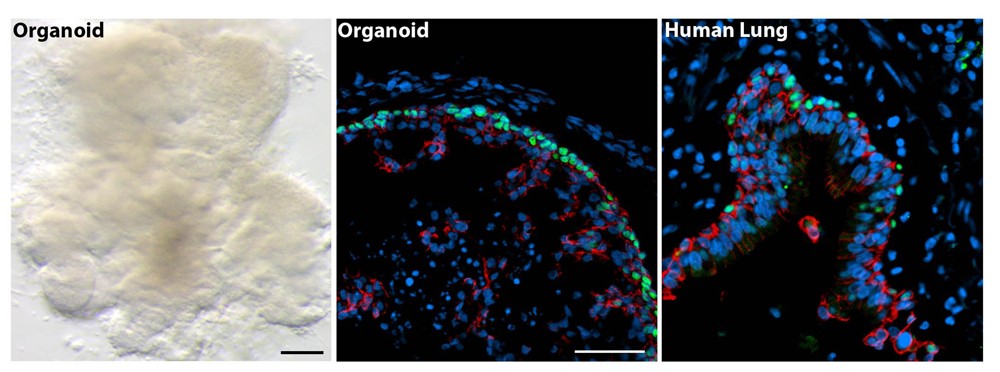
Lung
Researchers have grown 3D lung organoids in the lab that developed airway structures called bronchi and small lung sacs known as alveoli.
"These minilungs can mimic the responses of real tissues and will be a good model to study how organs form [and] change with disease, and how they might respond to new drugs," Jason Spence, co-senior study author and a professor of internal medicine, biomedical engineering and cell and developmental biology at the University of Michigan Medical School, said in a statement. The minilungs survived in the lab for more than 100 days.
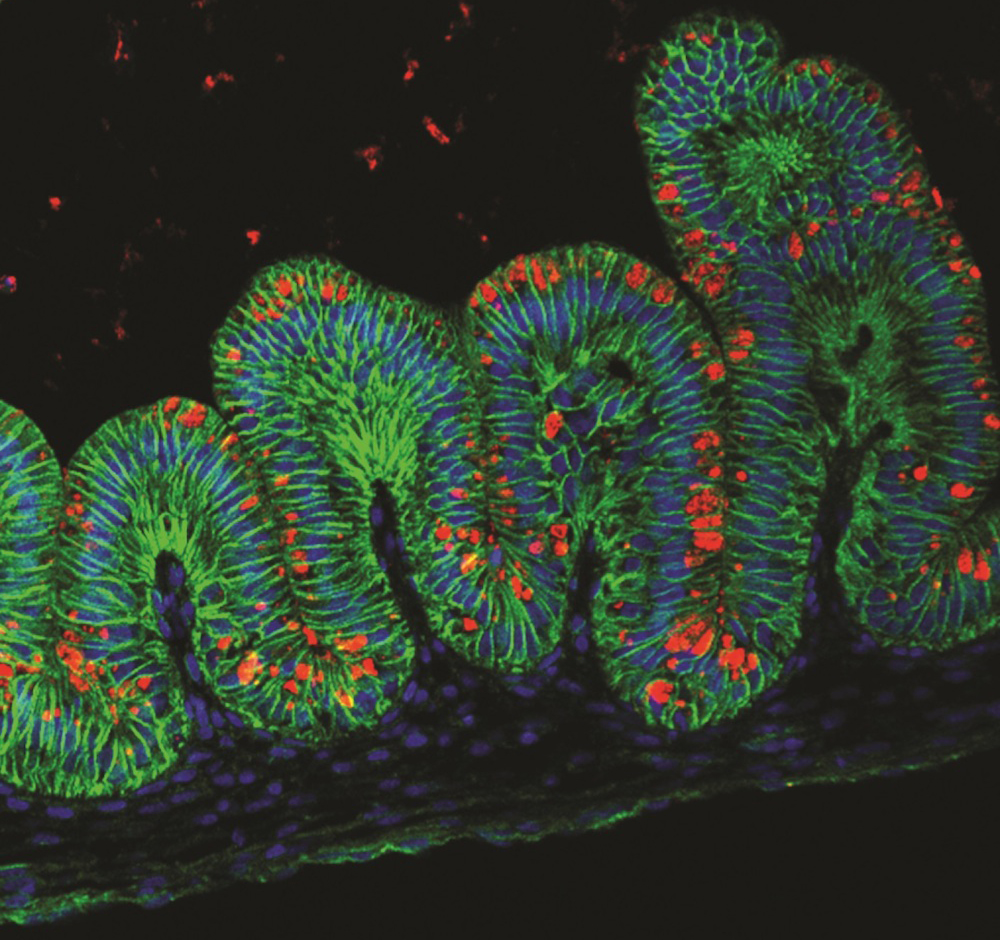
Stomach
In 2014, scientists grew mini stomachs in a lab dish. The organoids took around a month to develop and formed "oval-shaped, hollow structures" that had insides with folds like those seen in the human stomach, Jim Wells, one of the researchers who developed it and a professor of developmental biology at Cincinnati Children's Hospital Medical Center, told Live Science.
The tiny stomachs, which measured about 0.1 inches (3 millimeters) in diameter, will be especially helpful to scientists studying the effects of a certain species of bacteria called Helicobacter pylori that causes gastric disease, Wells said.
This is because H. pylori behaves differently in animal subjects than it does in humans, he said.
Vagina
In 2014, a study published in the journal The Lancet described the successful transplantation of lab-grown vaginas in four teenage patients between age 13 and 18. The organoids were created by nurturing the patients' cells on a vagina-shaped scaffold in the lab. They were transplanted into the patients with the hope of correcting a congenital defect in which the vagina and uterus were either missing or underdeveloped.
The teenagers were examined annually for eight years after the transplant surgery, during which time the organs functioned normally, allowing pain-free intercourse, the team reported.
Almost a decade later, scientists developed the world's first "vagina-on-a-chip". The device was around 1 inch (2.54 centimeters) long and contained live human cells which could be inoculated with bacteria that normally reside in the human vagina.
Penis
Scientists have also grown penile erectile tissue in the lab using cells from rabbits. The lab-grown penises, which were developed in 2014, could be transplanted into male rabbits, who went on to mate successfully. The aim is that these penile organoids could be given to men with groin injuries or congenital abnormalities, such as soldiers injured in combat.
However, at the time, the research was still in its experimental stage, with approval from the U.S. Food and Drug Administration (FDA) required for the team to extend its work and incorporate human tissue and subjects.
Ear
Scientists have 3D-printed human ears which were made from growing living cells around an ear-shaped mold for approximately three months. The mold was created by modeling a child's ear using 3D software and then sending the model to a 3D printer.
Researchers then seeded the 3D printed mold with a cocktail of living cow ear cells, as well as collagen from rat tails to support the cells' growth. The artificial ears were then implanted into rats for one to three months while scientists evaluated how the organs changed in size and shape as they grew. The next step will be to look at the use of human ear cells, rather than those from cows. The hope is to eventually create a replacement ear for children who are born with microtia, a congenital deformity in which the external ear does not develop properly, often leading to hearing loss.
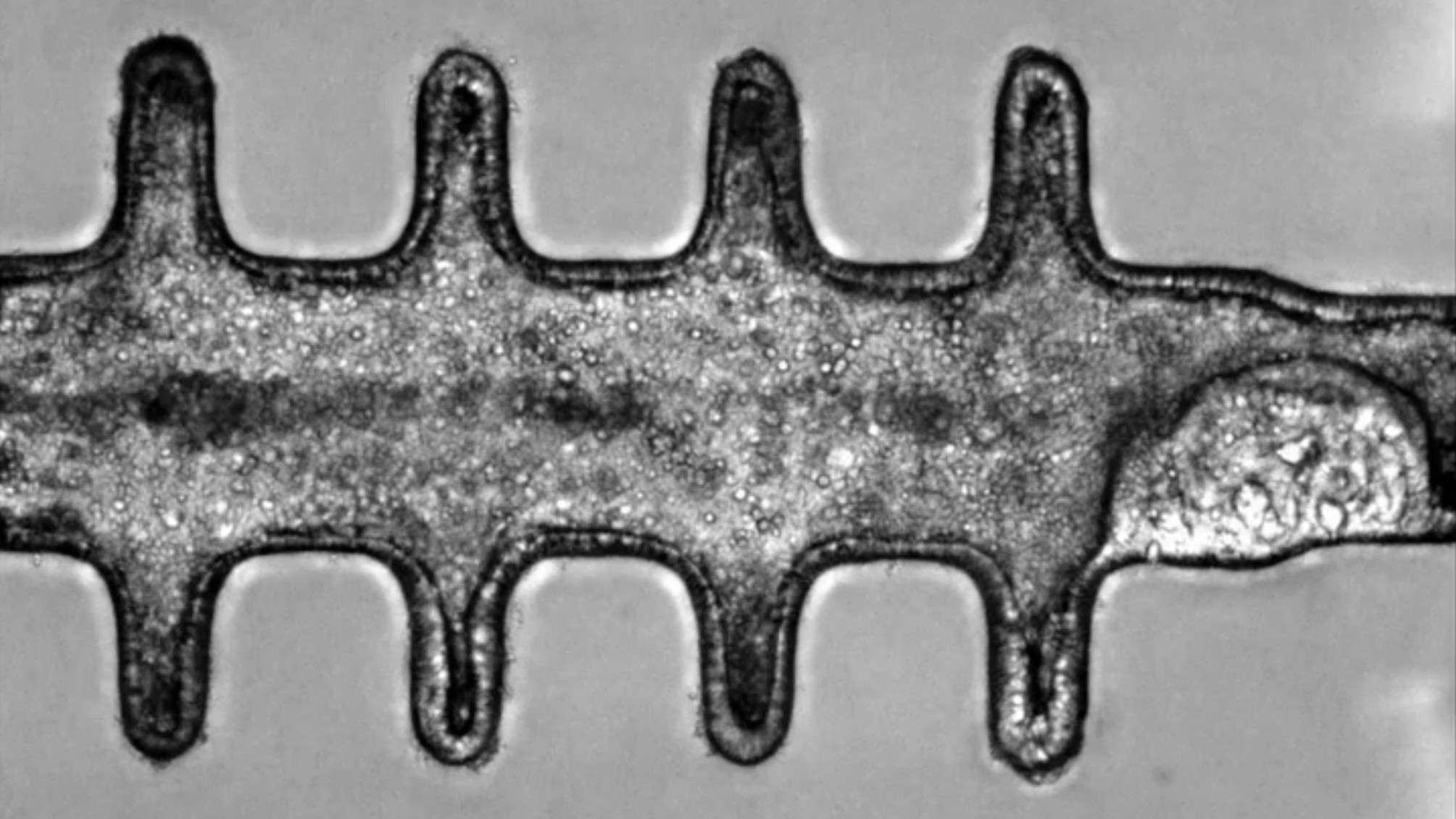
Colon
In 2024, scientists created realistic, 3D "minicolons" from mouse stem cells that were stimulated to mature in a lab dish using growth-inducing chemicals.
The team then triggered colorectal cancer in the organoids by switching on specific cancer-related genes in the tissue. These tumors could be grown for several weeks in a lab dish and resembled cancer seen in mice. This allowed the researchers to study the development of the disease in more detail than ever before.
Next, the team plans to grow these minicolons using cells from patients with colorectal cancer to make the work more applicable to humans.
Testicles
Miniature, 3D versions of testicles also make it to the list of body parts that scientists have grown in the lab.
The organoids were grown for the first time in 2024 from mouse cells with the help of growth-inducing chemicals. They survived in a lab dish for up to nine weeks and looked remarkably like the real thing. Researchers say these organoids could be used to study conditions in which testicular function is impeded, such as sex development disorders and male infertility.
Placenta
Scientists have also modeled the placenta in the lab in order to study how the organ develops during pregnancy and what causes serious complications to arise.
In 2018, scientists created 3D miniplacentas composed of a variety of cells and organ structures. The organoids even secreted hormones that could produce a positive result in an over-the-counter pregnancy test, researchers found.
Six years later, the same team used a slightly more developed model to identify proteins that are crucial for healthy placental development. These proteins influence blood flow and the implantation of the placenta in the uterus. Understanding more about their dysfunction could provide important insights into pregnancy disorders such as preeclampsia, the team said.
Conjunctiva
Researchers have grown a 3D model of the eye's clear, protective outer membrane — a structure known as the conjunctiva.
The organoid was created using stem cells from conjunctival tissue that had been provided by organ donors and patients who were having eye surgery. The organoid contained all the different types of cells that would normally be found in the human conjunctiva, including those that are needed to make mucus-rich tears. It even contained a type of surface cell that had not previously been found in the conjunctiva, but that had been tied to allergies.
With further development, researchers hope that the organoid will eventually be used to make replacement conjunctivae for people with eye burns or cancer, for instance.
Scientists have also developed a way to grow 3D models of the retina, or the photosensitive area at the back of the eye, in a quick and consistent way. These organoids could be used to develop new therapies for retinal degenerative diseases, the team says.
Blood vessels
Another addition to the list of body parts that have been grown in the lab is blood vessels. In 2024, scientists created a "blood-vessel-on-a-chip" which mimicked the shape of blood vessels and showcased how blood flows through them.
The 3D model contained cells that line human blood vessels, along with the physical framework that supports them.
The team hope that it will be used to study the potentially-fatal impacts of snake venom and accelerate the development of new antivenoms.
Tumors
Scientists have also modelled human tumors in the lab. In 2024, for instance, a research team grew organoids of glioblastomas, which are the most aggressive type of brain cancer. They used the models to quickly and accurately test how these kinds of tumors respond to CAR T-cell therapy — a treatment that involves re-programming a patients' immune system to attack cancerous cells.
Such models are helpful because it is difficult to measure exactly how a patient reacts to a given cancer treatment, at the cellular level, the team said in a statement. That's because you can't take regular tissue samples from the brain, and it is difficult to distinguish between tumor growth and treatment-related inflammation on magnetic resonance imaging (MRI) scans, they said.

Mindy Weisberger is a science journalist and author of "Rise of the Zombie Bugs: The Surprising Science of Parasitic Mind-Control" (Hopkins Press). She formerly edited for Scholastic and was a channel editor and senior writer for Live Science. She has reported on general science, covering climate change, paleontology, biology and space. Mindy studied film at Columbia University; prior to LS, she produced, wrote and directed media for the American Museum of Natural History in NYC. Her videos about dinosaurs, astrophysics, biodiversity and evolution appear in museums and science centers worldwide, earning awards such as the CINE Golden Eagle and the Communicator Award of Excellence. Her writing has also appeared in Scientific American, The Washington Post, How It Works Magazine and CNN.
 Live Science Plus
Live Science Plus