Inside Look at How Batteries Work (Infographic)
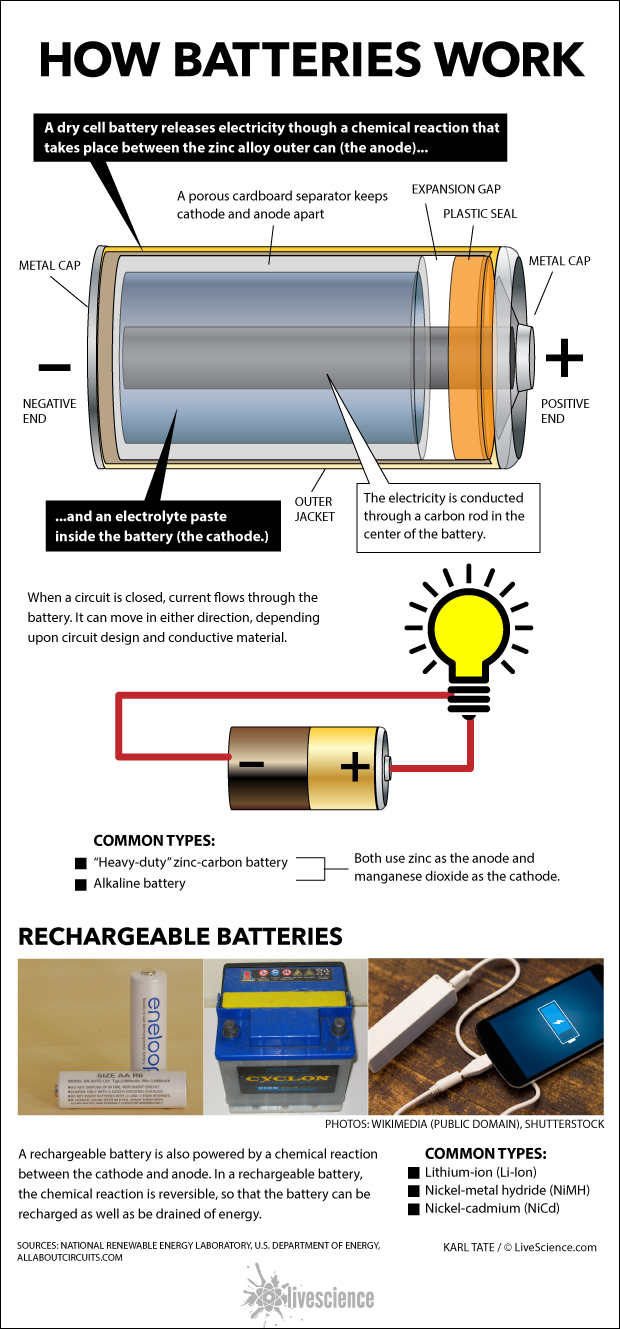
A dry cell battery releases electricity though a chemical reaction that takes place between the zinc alloy outer can (the anode) and an electrolyte paste inside the battery (the cathode.)
A porous cardboard separator keeps cathode and anode apart. The electricity is conducted through a carbon rod in the center of the battery.
When a circuit is closed, current flows through the battery. It can move in either direction, depending upon circuit design and conductive material.
A rechargeable battery is also powered by a chemical reaction between the cathode and anode. In a rechargeable battery, the chemical reaction is reversible, so that the battery can be recharged as well as be drained of energy.
Follow Live Science @livescience, Facebook & Google+.
Get the world’s most fascinating discoveries delivered straight to your inbox.

 Live Science Plus
Live Science Plus





