Strange Shrinking Material
Most materials expand when heated. They need the extra elbow room because the atoms inside jiggle around more when the temperature increases.
This stretching out can be damaging in certain circumstances, if the stuff is in an intricate computer part or used to fill your teeth, for example. A newfound substance could prove helpful.
Zirconium tungstate shrinks as it gets hotter. Discovered in 1996, this novel material surprised scientists because it exhibited its strange property, called negative thermal expansion, over a wide range of temperatures.
"No one understood why it didn't collapse into a denser structure, which would destroy the environment that sustains negative thermal expansion," says Zack Schlesinger from the University of California, Santa Cruz.
There are other materials that experience negative thermal expansion, but they lose it when the temperature changes too much. Now Schlesinger and his colleagues think they have found the reason for zirconium tungstate's stability in something called geometrical frustration.
The phenomenon is a bit like the age-old problem of trying to fit a square peg in a round hole.
"You can think of it as trying to tile a floor with pentagons," Schlesinger told LiveScience in a telephone interview.
Get the world’s most fascinating discoveries delivered straight to your inbox.
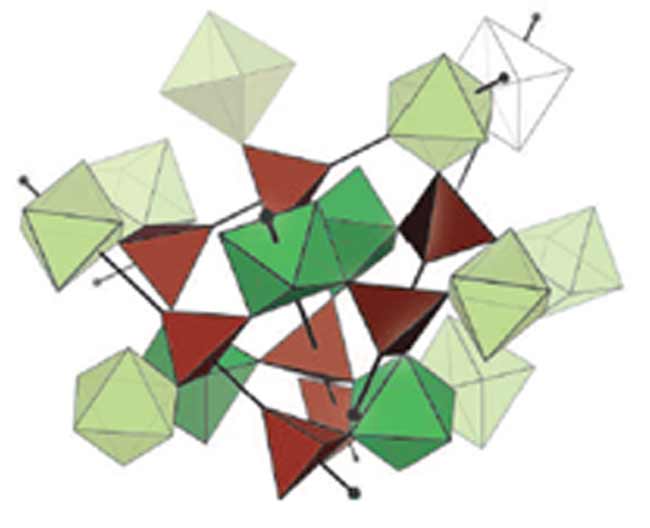
In the case of zirconium tungstate, the crystal structure can be divided up into four-sided tetrahedra and eight-sided octahedra. When the temperature rises, these geometric figures squeeze together, but they do not match up very well.
If there were no geometric frustration, zirconium tungstate would eventually rearrange itself and behave like most other materials. This at least is the hypothesis of Schlesinger and his colleagues, as presented in the Nov. 26 issue of Physical Review Letters.
But why the tetrahedra and octahedra squeeze together in the first place? Schlesinger said it has to do with the fact that they can vibrate back and forth with respect to each other.
In most solids, crystal structures only twist and tilt in response to heat. The pieces in zirconium tungstate do that as well, but they have one more way to move. In a sense, this allows them to fold in on each other - just not perfectly.
There are ceramics, like those used in some types of kitchen bakeware, which contract in one direction when heated, while expanding in another. Zirconium tungstate shrinks in all directions equally. If heated from negative 459.4 degrees Fahrenheit to plus 1,430 degrees, its volume decreases by about three percent.
Schlesinger says that this rate of contraction is comparable to the rate of expansion in common materials. For this reason, zirconium tungstate could be mixed with another substance, so that the two materials would offset each other when the temperature changed - thereby reducing strains.
Besides making electronic components that can handle more heat, zirconium tungstate could be used to make dental fillings that won't break when you drink hot tea after ice cream.

