These astonishing biobots can help neurons regrow — but researchers have no idea how
Tiny biological robots can move on their own, assemble into 'superbots' and encourage nerve cells regrow.
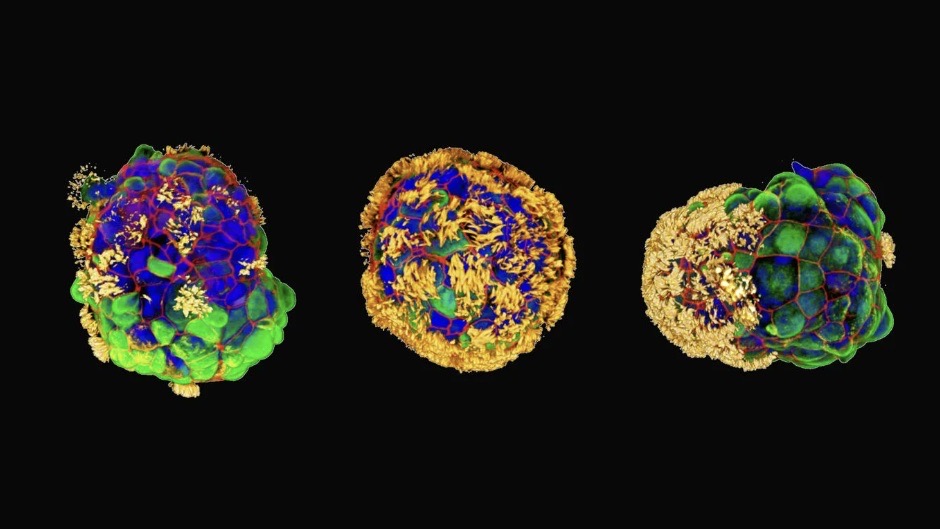
Scientists have created tiny, self-assembling robots made from human cells that could one day repair damaged skin and tissue.
These tiny biological machines, called Anthrobots, are made from human tracheal cells without any genetic modification. Lab dish experiments revealed they can encourage neurons, or nerve cells, to grow in damaged tissue.
The bots range in size, with the smallest no wider than a human hair and the largest about the size of a pencil tip. They assemble in clusters, which researchers called a "superbot". The scientists published their research on Nov. 21 in the journal Advanced Science.
In the study, the team used a metal rod to scratch a two-dimensional live layer of human neurons to simulate an open wound, before the Anthrobots clustered around it and triggered substantial neuron regrowth. The exact mechanism behind how Anthrobots encourage neurons to regrow, however, is not yet clear.
"The cellular assemblies we construct in the lab can have capabilities that go beyond what they do in the body," study lead author Michael Levin, a developmental and synthetic biologist at Harvard University, said in a statement.
Related: Tiny, shape-shifting robot could one day be used to perform surgery from inside the body
"It is fascinating and completely unexpected that normal patient tracheal cells, without modifying their DNA, can move on their own and encourage neuron growth across a region of damage. We're now looking at how the healing mechanism works, and asking what else these constructs can do."
Get the world’s most fascinating discoveries delivered straight to your inbox.
The team built each Anthrobot from a single human tracheal cell, which they then grew in a lab to form multicellular spheres called organoids. They encouraged the hairlike cilia normally found on tracheal cells to face outwards to help the organoids move around. The Anthrobots fell into different categories based on their size and movement patterns. The team then tested how well they'd perform in a therapeutic context.
Levin previously worked on another form of biobot called Xenobots. Derived from embryonic cells, these Xenobots navigated passageways, collected material, recorded information, healed themselves and even replicated for a few cycles. But Anthrobots go several steps further.
"Anthrobots self-assemble in the lab dish," Gizem Gumuskaya, a doctoral student at Tufts University and the scientist who created the Anthrobots, said in the statement.
"Unlike Xenobots, they don't require tweezers or scalpels to give them shape, and we can use adult cells — even cells from elderly patients — instead of embryonic cells. It's fully scalable — we can produce swarms of these bots in parallel, which is a good start for developing a therapeutic tool."
Using a patient's own cells to construct biobots reduces the risk of triggering an immune response or needing immunosuppressants, the authors said.
Anthrobots last between 45 and 60 days before breaking down and being reabsorbed by the body. They also don't reproduce, have not been gene edited, and can only survive in specific laboratory conditions outside the body. This means there's no risk of evolving beyond the existing safeguards, the researchers said.
The team would like to test the Anthrobots in other medical applications, including clearing plaque buildup in the arteries and repairing spinal damage or retinal nerve damage. They could even configure these tiny biobots to recognize bacteria or cancer cells, the researchers said.

Keumars is the technology editor at Live Science. He has written for a variety of publications including ITPro, The Week Digital, ComputerActive, The Independent, The Observer, Metro and TechRadar Pro. He has worked as a technology journalist for more than five years, having previously held the role of features editor with ITPro. He is an NCTJ-qualified journalist and has a degree in biomedical sciences from Queen Mary, University of London. He's also registered as a foundational chartered manager with the Chartered Management Institute (CMI), having qualified as a Level 3 Team leader with distinction in 2023.
 Live Science Plus
Live Science Plus





