Fluorescent flashes reveal the leaf-closing secrets of 'touch-me-not' plant
Flashy new videos of the plant shameplant (Mimosa pudica), which is renowned for its ability to rapidly fold up its leaves, reveal how chemical and electrical signals help to trigger its lightning reflexes.
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
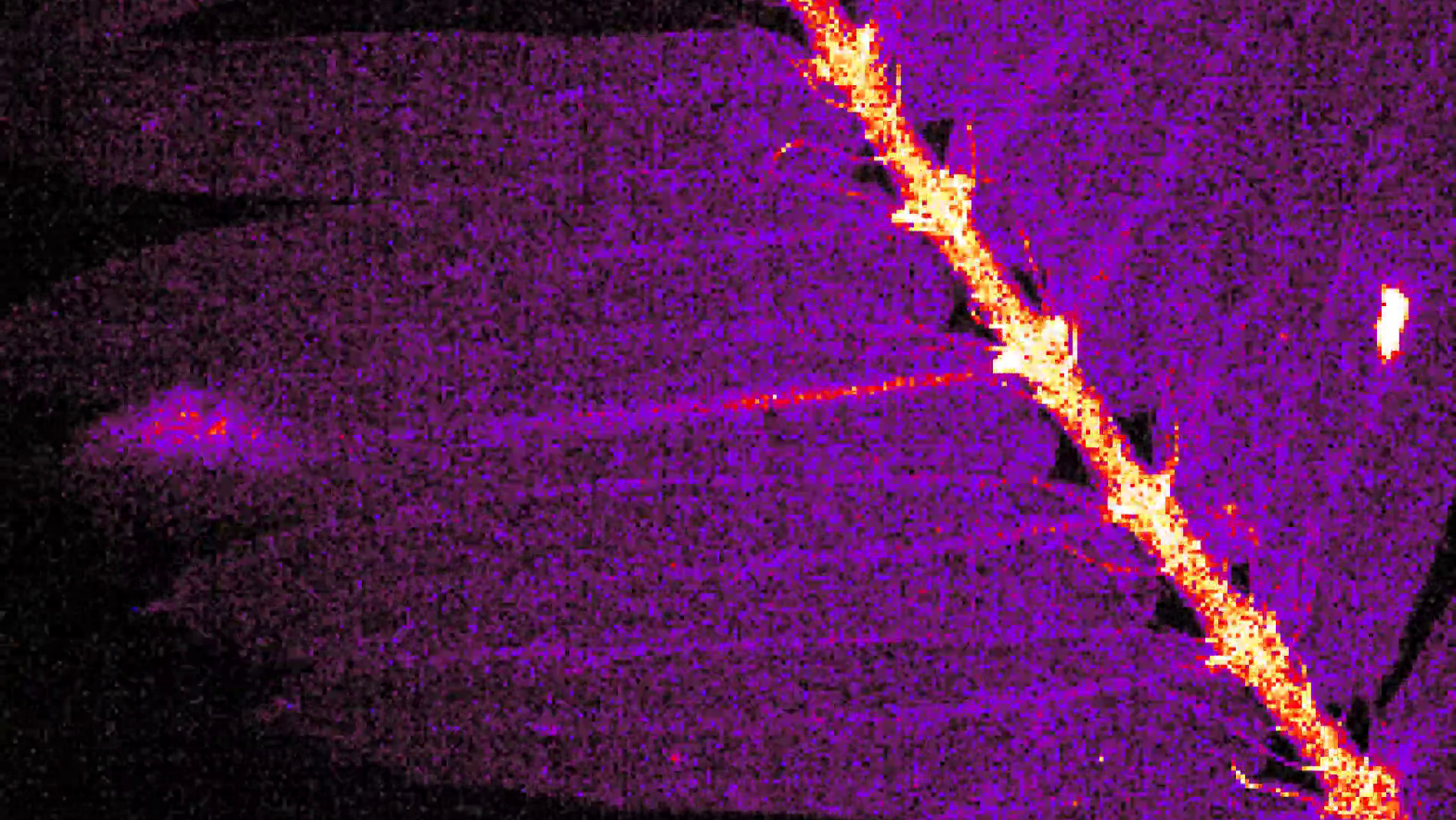
Flashes of light shoot along the spines of fluorescent-stained leaves from the "touch-me-not" plant as they fold up in striking new videos. The flashy footage has revealed how the plant closes up in a matter of seconds, despite lacking nerves and muscles.
The shameplant (Mimosa pudica), also known as the touch-me-not, is renowned for its ability to quickly curl up its leaves when they are touched by retracting the leaf's elongated, pine-like leaflets back toward its central spine. However, until now, the exact mechanisms behind this animal-like reflex have largely remained a mystery.
In a new study published Nov. 14 in the journal Nature Communications, a team of researchers created genetically-altered, fluorescent shameplants and then filmed their leaves as they curled up. The resulting footage revealed that both chemical and electrical signals moved in unison through the leaves and triggered the leaflets to be pulled back.
"Plants possess various communication systems that are normally hidden from view," study co-author Masatsugu Toyota, a plant physiologist at Saitama University in Japan, said in a statement. The best way to figure out how they work is to make them visible, he added.
In the new videos, parts of the leaves light up as action potentials — the electrical depolarizations of cell membranes — move through them. This is similar to how nerves work in animals but without specialized cells to channel the electrical energy, the signals travel more slowly through the plant's tissue.
Until now, researchers had suspected that either action potentials were the main signaling mechanism used by shameplants, but the videos reveal for the first time exactly how the signals are created. As cells depolarize, they release calcium ions that react with the fluorescent markers placed in mutated plants.
Get the world’s most fascinating discoveries delivered straight to your inbox.
The most noticeable flashes are those that consecutively light up along the center of the leaves. These are given off by tiny organs called pulvini, which pull back the leaflets toward the leaves' spines using changes in water pressure. However, fainter fluorescent signals (only visible when magnified) also travel along the leaflets from where a stimulus is detected to the closest pulvinus, before the folding organs start flashing. Once one pulvinus is activated, it then sends a signal to the adjacent pulvini, which creates a bright domino effect along the leaf's spine.
Scientists already knew about pulvini but, until now, they had no idea how quickly they contracted adjoining leaflets. The new videos show that the pulvini receive signals around 0.1 seconds before the leaflets contract, which is exceptionally fast for a plant, researchers wrote in the paper.
The new study also sheds light on why shameplants have evolved to close up their leaves.
The leading theory is that the leaves close up to protect themselves from hungry insects. In the study, researchers created additional genetic-variants of shameplants, which had no pulvini and therefore could not close up their leaves. The team then exposed the mutated and non-mutated plants to grasshoppers and found that the mutated plants had many more of their leaves eaten by the insects.
Other potential reasons for the shameplants to close up their leaves include reducing water loss or preemptively hiding from insects, but there is less evidence to support these ideas, the team wrote in their paper.

Harry is a U.K.-based senior staff writer at Live Science. He studied marine biology at the University of Exeter before training to become a journalist. He covers a wide range of topics including space exploration, planetary science, space weather, climate change, animal behavior and paleontology. His recent work on the solar maximum won "best space submission" at the 2024 Aerospace Media Awards and was shortlisted in the "top scoop" category at the NCTJ Awards for Excellence in 2023. He also writes Live Science's weekly Earth from space series.
 Live Science Plus
Live Science Plus