Peculiar parasitic fungi discovered growing out of the rectum of a 50 million-year-old fossilized ant
It is the oldest example of an ant infected by a fungal parasite ever found.

Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
Scientists have identified a new species of extinct parasitic fungus bursting from the backside of a 50 million-year-old ant, all perfectly preserved in amber.
In addition to the bulbous mushroom protruding from the ant's rectum, evidence of the freaky fungus can be seen throughout the body of its unlucky host. The ant likely died as a result of its fungal infection and was fortuitously fixed in tree resin (which fossilizes into amber) shortly afterward. It is the oldest example of a fungal parasite ever discovered in ants.
The researchers named the new species of fungi Allocordyceps baltica — Allocordyceps translates to "new genus" in Greek and baltica refers to the Baltic region where the amber was discovered.
Related: 6 (or so) ways fungi can help humanity
"These types of discoveries are extremely rare," George Poinar Jr., an entomologist at Oregon State University who helped pioneer the extraction of DNA from amber, told Live Science. "The amber resin contains chemicals that fixes cells and tissues and also destroys associated microbes that would normally decompose specimens."
Peculiar parasite
Parasitic fungi are hard to find and study due to their short life cycles, Poinar said. "But we all bear some fungal growth on our bodies," he added.
Insects are a great host for these types of parasites because they are "readily available and supply a rich source of nutrients," Poinar said.
Get the world’s most fascinating discoveries delivered straight to your inbox.
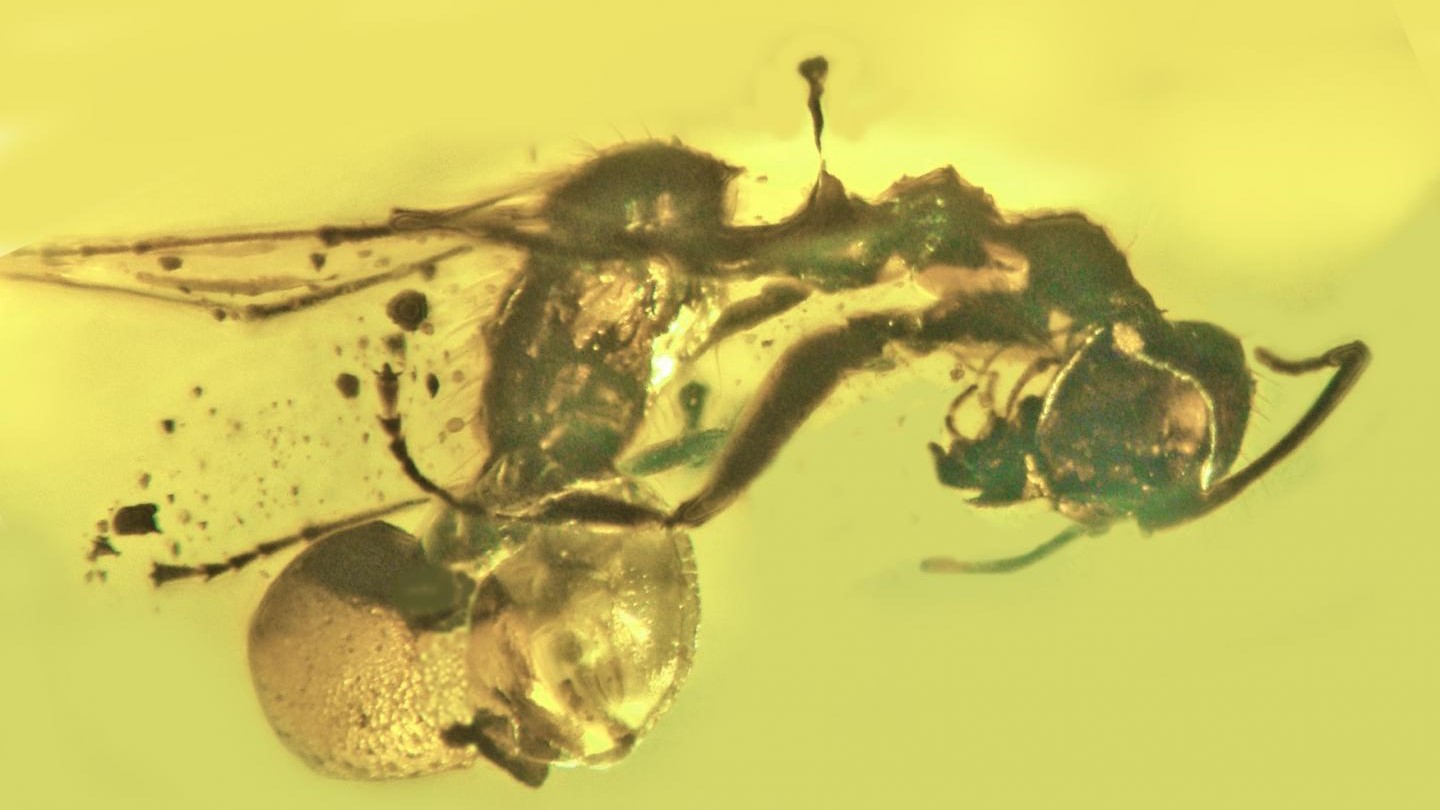
Carpenter ants of the genus Camponotus, like the one trapped in the amber, are common hosts of modern parasitic fungi of the genus Ophiocordyceps, which belong to the same order as A. baltica. "I was quite excited when I realized that these particular fungi extend back so far," Poinar said.
Although A. baltica are likely extinct today, its lineage could have evolved into modern-day Ophiocordyceps, Poinar said, although this has not yet been proved genetically.
Out the backdoor
The main difference between A. baltica and Ophiocordyceps is where their mushroom emerges from an ant. The mushroom, or ascomata, acts as the reproductive organ of the fungi, releasing spores into the environment. Ophiocordyceps fungi grow their ascomata around the neck and head of their host ants; the fungi hijack the host ants’ brain in a form of mind control, which the fungi use to force the ants to bite into plants where other carpenter ants lay their eggs. This enables the fungi to release their spores in areas with a high concentration of potential new hosts.
It is unclear why A. baltica grew its ascomata through the ant’s rectum instead, although Poinar suspects it may have allowed the fungus to keep its host alive for a longer period of time, meaning it had more time to distribute spores.
"The rectum is already open while the fungus would have to penetrate the head capsule to emerge through the head," Poinar said. "It would have allowed the ant to survive a few more days, since once the fungus enters the ant’s head the ant dies."
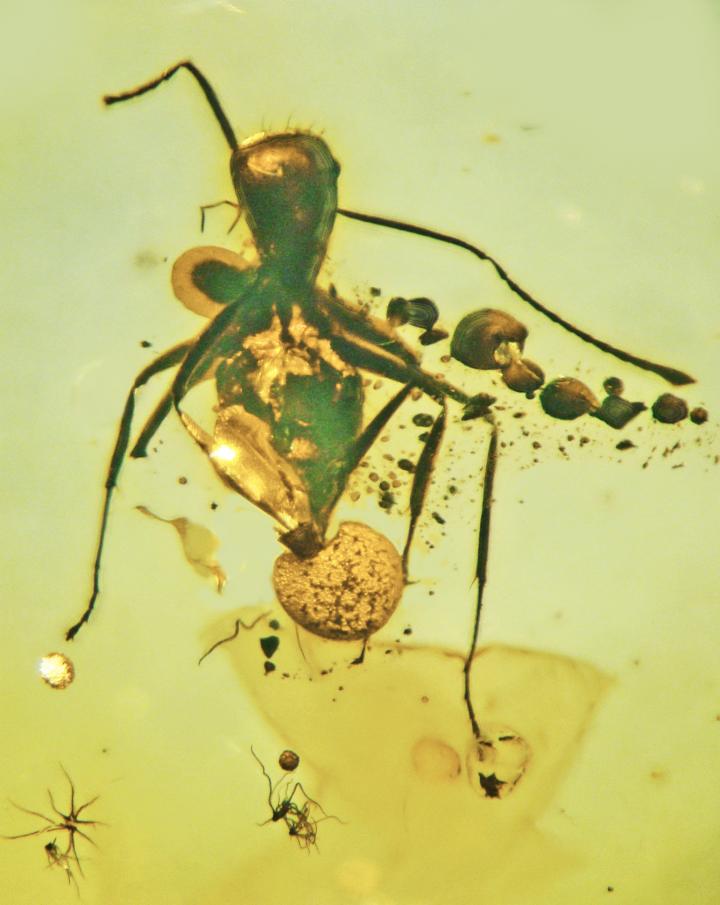
Nasty way to go
Although the reproductive ascomata emerges through the rectum of the fossilized ant, there is evidence that the fungus spread through that ant’s entire body. Stromata ― solid plates of the vegetative part of the fungus, known as mycelium ― can be seen protruding from the ant at the abdomen and the back of the neck; and the researchers also found sacs where the reproductive spores would have been produced in the abdomen and neck.
This would have "almost certainly" led to a slow and gruesome for the infected ant, Poinar said.
"As the hyphae [the branching filaments of mycelium] spread through the body, it would have been like a cancer," Poinar said, "but converting the tissues into fungal stages instead of cancer cells."
Originally published on Live Science.

Harry is a U.K.-based senior staff writer at Live Science. He studied marine biology at the University of Exeter before training to become a journalist. He covers a wide range of topics including space exploration, planetary science, space weather, climate change, animal behavior and paleontology. His recent work on the solar maximum won "best space submission" at the 2024 Aerospace Media Awards and was shortlisted in the "top scoop" category at the NCTJ Awards for Excellence in 2023. He also writes Live Science's weekly Earth from space series.
 Live Science Plus
Live Science Plus










