How are people cured of HIV? Here's everything you need to know
Only a few people have been cured of HIV, but scientists are working to develop cures that could be accessible to more of those infected.

Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
Editor's note: This article was last updated on July 27, 2023.
In the past 20 years, a handful of people have been cured of human immunodeficiency virus (HIV), the virus that causes AIDS, through intensive medical procedures.
Several more people have received the treatment and also appear to be HIV-free, but it's too soon to definitively declare these patients cured. For now, they're described as being in long-term remission, and their cases are considered "possible" cures. All these patients received stem cell transplants, with cells collected either from adult bone marrow or from umbilical cord blood.
Scientists reported the first definitive HIV cure in 2008, and since then, two more definitive cures and two possible cures have been reported. The most recent reports of such cases — one definitive cure and one possible cure — came out in early 2023.
Experts say these treatments may become more common in upcoming years as scientists better understand them. However, for now, these treatments are risky and largely inaccessible to the tens of millions of people living with HIV worldwide. Thankfully, drugs for HIV, called antiretroviral therapies (ART), can greatly extend HIV-positive people's lifespans and cut their risk of spreading the virus, but the medications must be taken daily and for life, can interact with other drugs and carry a small risk of serious side effects.
So scientists hope these exceptional cure cases will pave the way to new, more-accessible treatment strategies that will rid more people of the virus.
Here's what we know about curing HIV.
Get the world’s most fascinating discoveries delivered straight to your inbox.
What treatments can cure HIV?
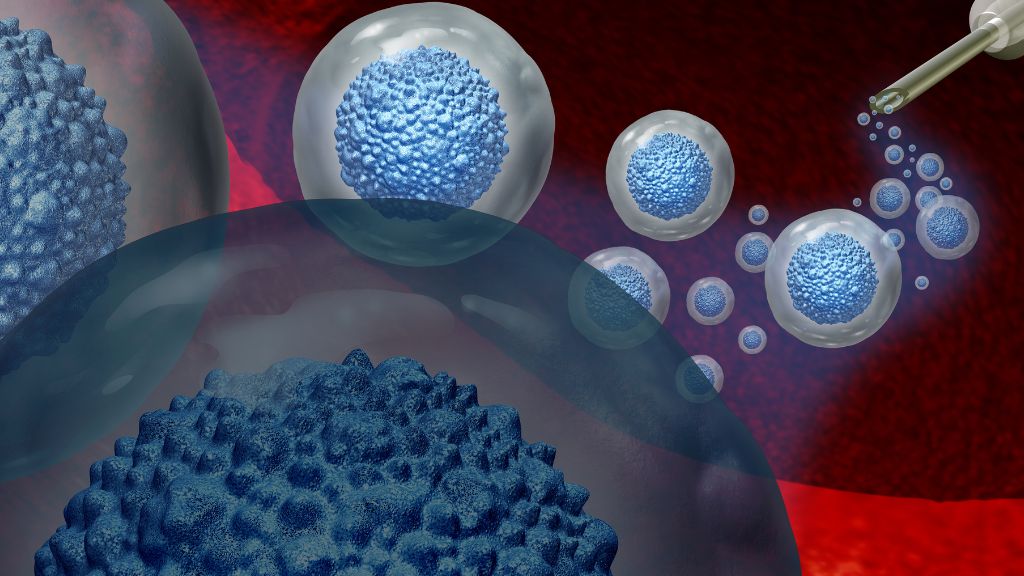
All of the people cured and potentially cured of HIV have been treated with stem cell transplants. In addition to being HIV-positive, all the patients had some form of cancer, typically acute myeloid leukemia or Hodgkin's lymphoma. These cancers affect white blood cells, a key component of the immune system, and can be treated with stem cell transplants.
In general, to simultaneously treat these patients' cancers and HIV, their doctors sought out stem cells from people with two copies of a rare genetic mutation: CCR5 delta 32. This mutation disables a protein on the cell surface called CCR5, which many HIV strains use to break into cells. The virus does this by first latching onto a different cell surface protein and changing shape; then, it grabs hold of CCR5 to invade the cell. Without CCR5, it's essentially locked out.
(Some less-common HIV strains use a different surface protein, called CXCR4, instead of CCR5, and some strains can use both, according to a 2021 review in the journal Frontiers in Immunology. Therefore, prior to their transplants, patients were screened to ensure that most or all of the virus in their body used CCR5.)
A donor with the CCR5 delta 32 mutation was found for all but one of the patients who entered long-term remission from HIV following a stem cell transplant. His mysterious case is still being investigated.
To prepare for the transplant, the patients underwent aggressive radiation or chemotherapy to wipe out the cancerous and HIV-vulnerable T cells — a type of immune cell — in their bodies. This weakened patients' immune systems until the transplanted stem cells could produce new, HIV-resistant immune cells. For some time post-transplant, patients also took immune-suppressing drugs to avoid graft-versus-host disease (GVHD), where the donor-derived immune cells attack the body.
Most patients received stem cells taken from the bone marrow of adult donors. These cells must be carefully "matched," meaning both the donor and recipient must carry specific proteins, called HLAs, in their body tissues. An HLA mismatch can result in a catastrophic immune reaction.
One patient — the first woman to undergo a stem cell transplant for HIV and enter long-term remission — received stem cells from umbilical cord blood that had been donated at the time of a baby's delivery. These immature cells adapt more easily to a recipient's body, so the patient had to be only "partially matched." She also received stem cells from an adult relative, to help bolster her immune system as the umbilical cells took over.
Because umbilical cord stem cells don't need to be a perfect match and they're easier to source than bone marrow, such transplants could potentially be offered to more patients in the future.
However, HIV-positive patients shouldn't undergo the risky procedure unless they have another disease that requires a stem cell transplant, Dr. Yvonne Bryson, director of the Los Angeles-Brazil AIDS Consortium at the University of California, Los Angeles and one of the cured patient's doctors, said at a March 2023 news conference.
Who was the first person cured of HIV?
The first person cured of HIV was initially called the "Berlin patient," because he'd been treated in Berlin, Germany. In 2010, he revealed his identity.
American Timothy Ray Brown was diagnosed with HIV in 1995 while attending a university in Berlin and started ART to reduce the amount of HIV in his body. In 2006, Brown was diagnosed with acute myeloid leukemia, and in 2007, he received radiation therapy and a bone marrow transplant to treat the disease. Brown's doctor saw this as an opportunity to treat his patient's leukemia and HIV at the same time.
Brown was HIV-free after the radiation and transplant, but his cancer later returned and he required a second transplant in 2008. That year, researchers announced that the "Berlin patient" was the first person cured of HIV.
Brown remained free of HIV through the end of his life. He died of cancer in 2020 at age 54, after his leukemia returned and spread to his spine and brain.
How many people have been cured of HIV?
As of March 2023, three people had been cured of HIV and two more were in long-term remission. In July 2023, researchers announced that an additional person was in long-term remission following a stem cell transplant, bringing that number to three.
In addition to Timothy Ray Brown, the cured individuals include the London patient, later revealed to be Adam Castillejo; and the anonymous Düsseldorf patient.
The three possible HIV cures include a man known as the City of Hope patient; the New York patient, the first woman to receive the treatment; and a man known as the Geneva patient, whose case is unusual and being closely monitored. (The City of Hope patient revealed his name — Paul Edmonds — on April 3, 2023, having received his transplant in February 2019 and stopped ART in March 2021.)
At present, there's no official distinction between being cured and being in long-term remission from HIV, Dr. Deborah Persaud, who helped oversee the New York case and is the interim director of pediatric infectious diseases at Johns Hopkins, said at a March 2023 news conference.
"[The Düsseldorf patient] was likely the second person to be cured, but the team was really conservative, and stopped antiretroviral therapy after several years, and waited a long time to conclude that he was cured," Dr. Steven Deeks, an HIV expert and professor of medicine at the University of California, San Francisco, who was not involved in the patient's case, told Live Science in an email.
The Düsseldorf patient was treated in 2013, continued ART for nearly six years and has now been off the medication for more than four. Meanwhile, Castillejo received his transplant in 2016, stopped ART a little over a year later and was confirmed cured in 2020, before the Düsseldorf patient.
What can we learn from HIV cures?
These cases provide information about how the body changes after a curative transplant as well insight into future strategies to cure HIV.
Scientists have found that, even post-transplant, supersensitive tests pick up "sporadic traces" of HIV DNA and RNA (a molecular cousin of DNA needed to build proteins). However, these viral remnants cannot replicate, said Dr. Björn-Erik Ole Jensen, a senior physician at the University Hospital of Düsseldorf who ran exhaustive tests on such remnants from the Düsseldorf patient.
That means none of these viral traces could make copies of themselves, he told Live Science. Doctors involved in the other cure cases ran similar tests, and got the same result.
Changes in the immune system might be a better measure of how well a transplant has worked, Jenson told Live Science. For about two years post-transplant, the Düsseldorf patient carried immune cells that reacted to HIV-related proteins, meaning they'd encountered and stored a "memory" of the virus.
"But over time, these responses faded away," Jenson said, as the reservoir of functional HIV dwindled to nothing. This change in immune activity was a convincing sign that the Düsseldorf patient could stop ART, he added.
Are scientists researching other ways to cure HIV?
Scientists are working on alternative treatments that can trigger these same changes in the body without relying on donor stem cells, Jenson said. By avoiding stem cell transplants, future treatments might eliminate the need for harsh chemotherapy, radiation and immunosuppressants, and the risk of GVHD.
Some research groups are developing an HIV cure based on a modified cancer therapy, in which they take some of a patient's immune cells, delete the CCR5 receptor and make the cells reactive to HIV proteins before returning them to the body.
Another potential cure strategy involves gene therapies that edit the DNA of cells within the body, to delete the gene for CCR5 or to prompt cells to make proteins that block or disable CCR5. Some researchers are developing ways to target CXCR4.
"With the revolution of gene editing now occurring in other areas of medicine, we may someday be able to do this with a single shot," Deeks said. These approaches are still being tested in lab dishes and animals, so scientists don't yet know how they'd work in humans, Jenson noted.
Nevertheless,"I think there is hope."

Nicoletta Lanese is the health channel editor at Live Science and was previously a news editor and staff writer at the site. She holds a graduate certificate in science communication from UC Santa Cruz and degrees in neuroscience and dance from the University of Florida. Her work has appeared in The Scientist, Science News, the Mercury News, Mongabay and Stanford Medicine Magazine, among other outlets. Based in NYC, she also remains heavily involved in dance and performs in local choreographers' work.
 Live Science Plus
Live Science Plus