Most melatonin gummies have higher doses than what's on the label
A new analysis reveals that most melatonin gummies sold in the U.S. contain more of the hormone than noted on their labels.
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
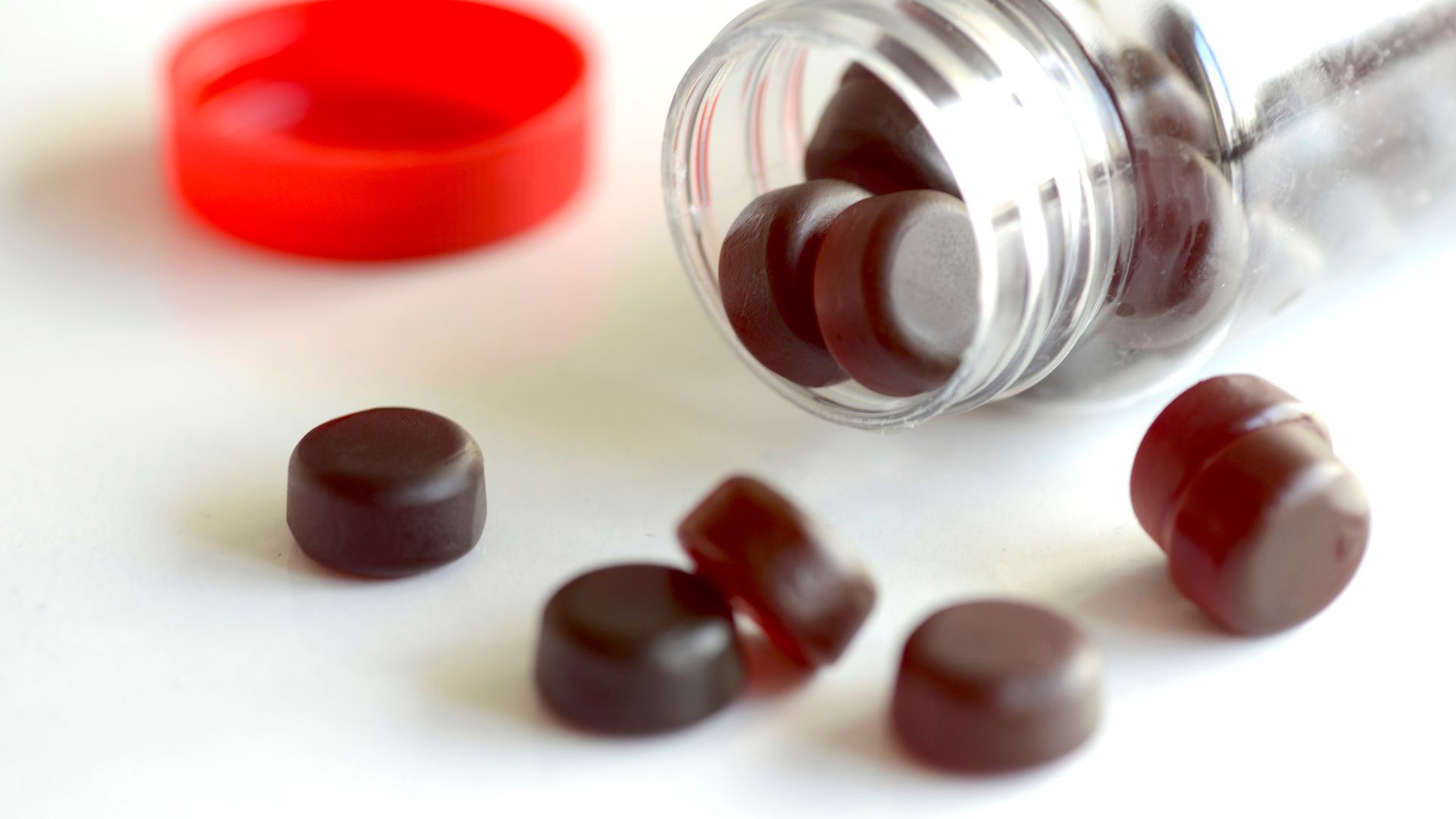
Most melatonin gummy supplements sold in the U.S. are inaccurately labeled, often containing higher quantities of the sleep-promoting hormone per serving than declared on the packaging, a new study finds.
The findings, published April 25 in the journal JAMA, mean that people who take the gummies are consuming uncertain amounts of melatonin, the researchers said. The results have particular implications for children, who may accidentally ingest the products, or who may be given them by parents who hope it will help them sleep, without understanding the potential risks.
Children's size puts them at greater risk of an overdose of melatonin than adults; overdose can lead to symptoms such as drowsiness, headache, dizziness, agitation and nausea, according to the National Institutes of Health (NIH). In rare cases, children who overdosed on melatonin have required hospitalization and experienced symptoms like respiratory failure or seizures, according to a study described in a 2022 Morbidity and Mortality Weekly Report (MMWR).
The doses found in the new study may not be dangerous to an adult taking one serving; but "if you're a younger, pre-teen taking a lot to go to sleep, or you're a school-age kid or toddler getting [into] these, that's where some really serious harm could occur," study lead author Dr. Pieter Cohen, a general internist at the Cambridge Health Alliance and an associate professor of medicine at Harvard Medical School, told Live Science.
Related: Weighted-blanket use may boost sleep hormone melatonin, small study hints
Melatonin is a hormone produced by the brain that helps regulate the body's circadian rhythms, or processes that follow a 24-hour cycle, including the sleep-wake cycle. In the U.S., melatonin is available as a dietary supplement, which means it is regulated less strictly by the Food and Drug Administration (FDA) compared with prescription drugs, according to the NIH.
In recent years, there has been a rise in the incidence of children ingesting too much melatonin, either accidentally or intentionally. The 2022 MMWR found that calls to U.S. poison control centers regarding pediatric ingestion of melatonin increased 530% from 2012 to 2021. Of the 260,000 reported cases, about 27,800 required medical treatment; 4,100 required hospitalization and 290 were admitted to the ICU.
Get the world’s most fascinating discoveries delivered straight to your inbox.
The MMWR findings prompted Cohen and his colleagues to examine melatonin supplements in the U.S. They chose to focus on gummies because this formulation may be more attractive to children.
The researchers used the NIH's Dietary Supplement Label Database, a governmental database of dietary supplements marketed in the U.S., to identify brands of melatonin gummies. They identified 30 brands and were able to purchase 26 for analysis. One of the purchased brands did not list melatonin on the actual bottle label and was excluded.
In the 25 remaining products, the team found that the actual amount of melatonin ranged from 74% to 347% of what was listed on the label, except in one case where there was no melatonin in the product. The majority of products — 88% — were inaccurately labeled, and 84% contained more melatonin than indicated.
The actual amount of melatonin found in the products ranged from 1.3 mg to 13.1 mg. Doses on the higher end of this range, between 5 mg and 10 mg, could be too much for a young child, Cohen said. Many children respond to doses as low as 0.5 mg to 1 mg, according to the American Academy of Pediatrics.
The study also revealed that five of the melatonin gummies' labels listed cannabidiol (CBD), a non-psychoactive compound found in marijuana. This finding was surprising because the FDA does not allow CBD to be sold as a dietary supplement, and yet, CBD was listed as an ingredient in these gummies on the NIH label database, Cohen noted.
(Since 2018, CBD has been legal in the U.S. if it is derived from hemp, according to the Centers for Disease Control and Prevention. However, the FDA has not approved CBD as a supplement or food additive, so it remains illegal to sell CBD in this form.)
The amount of CBD in the gummies ranged from 104% to 118% of what was listed on the label. One supplement didn't contain any melatonin but contained 31 mg of CBD.
"Given these findings, clinicians should advise parents that pediatric use of melatonin gummies may result in ingestion of unpredictable quantities of melatonin and CBD," the authors wrote in their paper. (Potential side effects from CBD, for children and adults, may include liver damage, drowsiness, diarrhea and irritability, according to the CDC.)
Melatonin "should not be treated as a warm glass of milk" to help children sleep, but rather, "it should be treated like a medication," Cohen said. This means keeping the product in a secure place and being careful about the dosage.
Caregivers should consult a doctor to see if melatonin would be appropriate for their child's specific sleep problem, he said. For example, melatonin has sometimes been found to be beneficial for children with neurodevelopmental disorders such as autism or ADHD who have trouble sleeping, and so doctors may recommend the hormone in these cases.
If melatonin is deemed an appropriate treatment, caregivers should seek out products that have undergone third-party testing to verify the product's quality, such as those that have been verified by the United States Pharmacopeia, or USP, which will be marked on the product's label, he said.

Rachael is a Live Science contributor, and was a former channel editor and senior writer for Live Science between 2010 and 2022. She has a master's degree in journalism from New York University's Science, Health and Environmental Reporting Program. She also holds a B.S. in molecular biology and an M.S. in biology from the University of California, San Diego. Her work has appeared in Scienceline, The Washington Post and Scientific American.
 Live Science Plus
Live Science Plus










