CRISPR used to 'reprogram' cancer cells into healthy muscle in the lab
In a new study, stopping skeletal-muscle cancer cells from making a specific protein forced them to turn into healthy muscle cells.
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
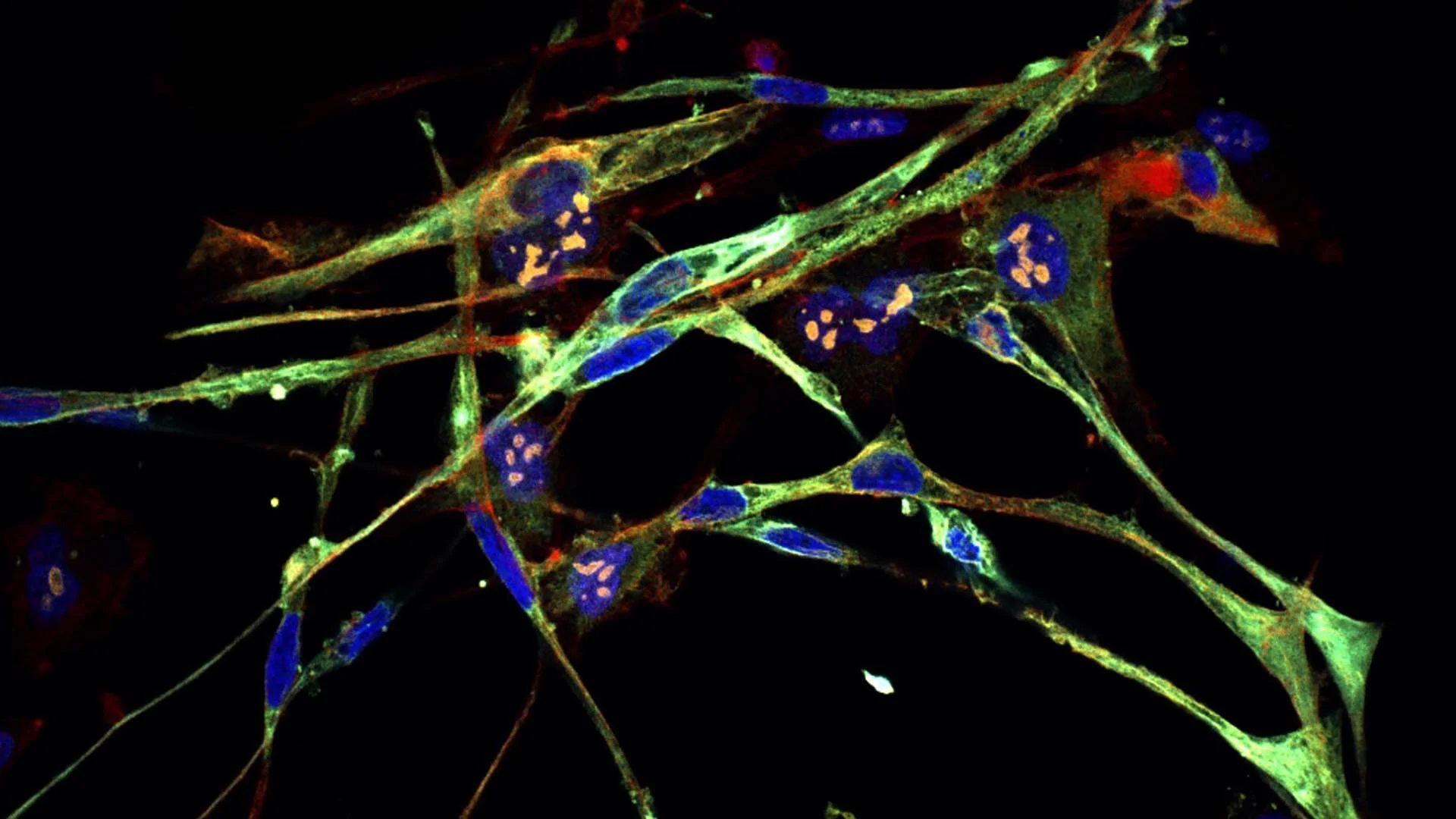
Scientists have transformed cancer cells into healthy muscle tissue in the lab using CRISPR gene-editing technology — and they hope new cancer treatments can be built on the back of this experiment.
In a study published Aug. 28 in the journal PNAS, researchers found that disabling a particular protein complex in cells of rhabdomyosarcoma (RMS) — a rare cancer in skeletal muscle tissue that mainly affects children under age 10 — in the laboratory causes the tumor cells to turn into healthy muscle cells.
Although the research is still in its early days, this process of "resetting" cancer cells into healthy cells, broadly known as differentiation therapy, has already been tested in other types of cancer, such as bone and blood cancer. Four drugs have been approved by the U.S. Food and Drug Administration (FDA) to treat the latter disease and generally work by inhibiting a specific protein in the cancer cells.
The protein complex pinpointed in the new research could serve as a target for such a therapy, the study authors wrote, and with further development, it could be a promising new treatment option for patients with RMS, which is normally treated with surgery, radiation and chemotherapy.
"This technology can allow you to take any cancer and go hunting for how to cause it to differentiate," or cause it to stop multiplying uncontrollably and turn into normal, noncancerous cells, Christopher Vakoc, lead author and professor at Cold Spring Harbor Laboratory, said in a statement. "This might be a key step toward making differentiation therapy more accessible."
Related: Meet 'Fanzor,' the 1st CRISPR-like system found in complex life
Differentiation is a process in which stem cells divide and form the various types of cells in the body, such as muscle or fat cells, which each have a unique pattern of gene expression that enables them to carry out particular functions. In RMS, however, patients have genetic mutations that cause their cells to make a specific protein, called PAX3-FOXO1, which stops differentiation from happening in skeletal muscle cells. So instead of turning into muscle, the cells form a mass of cancerous tissue.
Get the world’s most fascinating discoveries delivered straight to your inbox.
In the new study, the researchers used CRISPR to disable, or "knock out," different genes to see which ones make proteins that work together with PAX3-FOXO1 to stop RMS cells from differentiating. Their analysis revealed that, if RMS cells lose the ability to make nuclear factor Y (NF-Y) — a protein that regulates gene expression — the cells instead differentiate into muscle cells. Knocking out PAX3-FOXO1 directly has the same effect.
"The tumor loses all cancer attributes," Vakoc said in the statement. "They're switching from a cell that just wants to make more of itself to cells devoted to contraction."
Although deactivating PAX3-FOXO1 and NF-Y has similar effects, the researchers discovered that the proteins don't physically interact with each other. Instead, in RMS cells, NF-Y switches on the genes needed to make PAX3-FOXO1 by binding to a specific sequence of DNA. So by blocking NF-Y, the researchers also blocked production of PAX3-FOXO1.
The findings are still a long way from being translated into a treatment for RMS. However, drugs that inhibit NF-Y are already being developed, including those that stop the protein complex from forming or binding to DNA.
One hurdle that will need to be overcome is that NF-Y also regulates important processes in healthy cells, such as metabolism and the cell cycle, the series of steps that cells go through as they grow and divide. However, Vakoc and team hypothesize that because RMS cells are "highly sensitive" to changes in PAX3-FOXO1 expression, there could be a "window of opportunity" in which a drug inhibits NF-Y long enough for RMS cells to differentiate but not so long that healthy tissues get damaged. More research will be needed to confirm that this is a viable treatment strategy, they wrote.

Emily is a health news writer based in London, United Kingdom. She holds a bachelor's degree in biology from Durham University and a master's degree in clinical and therapeutic neuroscience from Oxford University. She has worked in science communication, medical writing and as a local news reporter while undertaking NCTJ journalism training with News Associates. In 2018, she was named one of MHP Communications' 30 journalists to watch under 30.
 Live Science Plus
Live Science Plus










