New Research Points to Cancer Drugs in Lower Doses
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
Amino acids, those not employed in creating protein and life, can improve a cancer therapy protein's activity by more than 30-fold.
Scientists say this opens the door to experimenting with these novel compounds throughout proteins and, in the future, in organisms, possibly creating drugs that work better but have fewer side effects.
Life normally uses 20 amino acids as the building blocks for proteins, but a vast number of other amino acids exist. By using these amino acids--known as unnatural amino acids--in proteins, "we have access to many more shapes and charges as compared to nature," researcher Ryan Mehl, a biochemist at Franklin and Marshall College in Lancaster, Penn., told LiveScience.
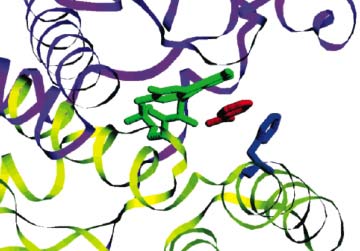
No unnatural amino acids until now had been shown to improve the activity of any enzymes, which are proteins essential to life's activities. Mehl and his colleagues experimented with the enzyme nitroreductase, which helps activate cancer therapy drugs.
One amino acid within nitroreductase is key to it binding to the drugs it activates. The researchers tried replacing this amino acid with all the other natural ones and eight unnatural amino acids, with the resulting enzyme variants generated by bacterial cells in lab dishes.
Using the unnatural amino acids, the scientists created a nitroreductase variant more than 30 times more efficient than normal. It was also more than twice as good as the best variant that used a natural amino acid replacement.
In the future, this could mean patients could use reduced amounts of the cancer drug, which might cut down on bad side effects. Improving enzymes for commercial use should be feasible in the near future, Mehl said. He and his colleagues report their findings in the Journal of the American Chemical Society.
Get the world’s most fascinating discoveries delivered straight to your inbox.
"We can improve upon nature by working with only one site--just imagine the potential of using these amino acids throughout a protein or organism," Mehl said. While "evolving a whole protein instead of one site will be technically challenging but is currently feasible," he cautioned "evolving unnatural organisms in a convincing way might be a couple years off."

 Live Science Plus
Live Science Plus










