Substitute Tissue Could One Day Fix Damaged Hearts
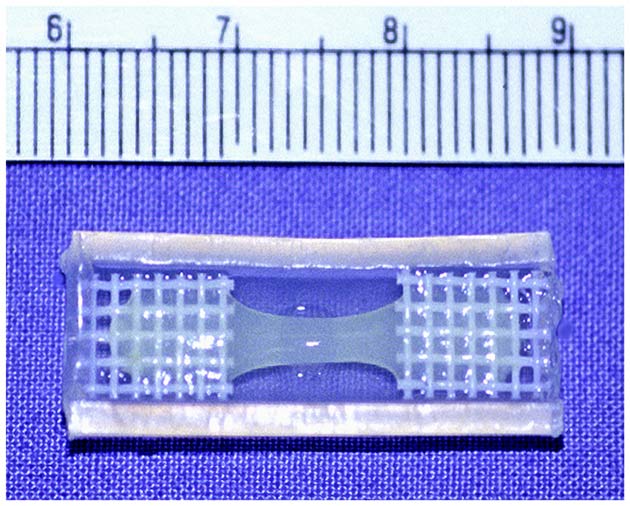
Scientists have succeeded for the first time in creating patches of substitute tissue that can conduct the electricity needed to pump the heart.
The idea is to surgically implant tissue created from a patient's own cells to fix faulty electrical signaling in the heart, rather than relying solely on pacemakers, which are fairly reliable in adults but can cause problems over the long term, especially in children as they grow.
So far, the new approach works in adult rats and is being tested in larger juvenile animals, such as lambs.
How it's done
The team from Children's Hospital Boston creates the tissue by mixing precursor cells isolated from skeletal muscle with collagen, the gelatinous compound that makes up bones and other connective tissues in the body.
The engineered material starts out with the consistency of hair gel when it is cast into molds the size of a big paper clip. It hardens to the consistency of a gummy bear, only less dense, but still able to support sutures, or stitches. Scientists then cut out smaller pieces for immediate implantation, said Douglas Cowan, a cell biologist who led the team.
The tissue could eventually be used to help patients with a condition called complete heart block, which disrupts the electrical signals in heart tissue and prevents signals from passing from the heart's upper chambers (atria) to the lower chambers (ventricles), leading to heart failure. In healthy hearts, electrical impulses synchronize the beating of the chambers as impulses move between the atria and ventricles via something called the atrioventricular (AV) node.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Advantages
The advantage to using cells from skeletal muscle rather than the heart itself is that heart muscle doesn't regenerate well—a heart attack deprives a region of the heart of oxygen, and the cells die—especially in newborns whose hearts are so tiny that they have no spare tissue for a biopsy. Also, cells from skeletal muscle can go without a good blood supply for a relatively long period of time, Cowan told LiveScience.
The new tissue is a biological substitute for the AV node.
"The idea was that rather than using a pacemaker, we could create an electrical conduit to connect the atria and ventricles," said Cowan, whose recent work on the new tissue was published in the American Journal of Pathology.
The challenge
In recent years, scientists whose work bleeds into the field of engineering have figured out how to use patients' own cells and other animal tissues to help grow new tissues to replace sick body parts such as blood vessels, skin, cartilage, bone, stomachs, and bladders. A big challenge is getting veins and arteries to supply blood from the body's natural tissues to newly implanted tissues.
Cowan's team was able to get their engineered heart tissue to make those vascular connections.
Complete heart block occurs in about 1 in 22,000 births. It also afflicts people with congenital heart disease.
Pacemakers, the current standard treatment for patients with faulty electrical signaling in their hearts, are implanted into patients' chests or abdomens, but they often fail over time, particularly in infants and small children who must undergo surgery after surgery to keep the pacemakers working as their bodies grow.
In children, pacemakers can result in heart perforation and blood clots. And the leads must be replaced frequently, requiring many repeat operations. In very small babies, the pacemaker leads must be placed in precise positions on the heart's surface, making it even more difficult for surgeons to succeed.
Good integration
In the lab, Cowan's team demonstrated that their fabricated tissue starts beating when stimulated electrically and the muscle cells produce proteins that facilitate electrical connections by channeling charged molecules from cell to cell.
Implanted in rats between their right atrium and left ventricle, the cells integrated well with surrounding heart tissue. And the implants kept working for the rest of the animals' lifespan (about three years).
Cowan and his colleagues have more work to do, such as trying to create tissue with electrical timing more like a natural AV node, possibly by using stem cells derived from muscle or bone marrow.
Down the line, the new tissue could be used in conjunction with pacemakers, which people think of as fairly reliable. The fabricated AV node tissue must be even more reliable for people to be willing to make the switch, Cowan said.
"We really need to have this function in every single patient that it would be implanted in," he said.
- Body Quiz 1: The Parts List
- Body Quiz 2: How the Parts Fit
- Good-Hearted Women Fail to Deal with Bad Hearts
- Doctor's Advice: Have Your Heart Attack During Normal Business Hours
- Fantastic Voyage to Save the Heart
- How Heart Attacks Strike
Robin Lloyd was a senior editor at Space.com and Live Science from 2007 to 2009. She holds a B.A. degree in sociology from Smith College and a Ph.D. and M.A. degree in sociology from the University of California at Santa Barbara. She is currently a freelance science writer based in New York City and a contributing editor at Scientific American, as well as an adjunct professor at New York University's Science, Health and Environmental Reporting Program.
 Live Science Plus
Live Science Plus






