'Coffee-Ring Effect' Could Reveal What's in Your Tap Water
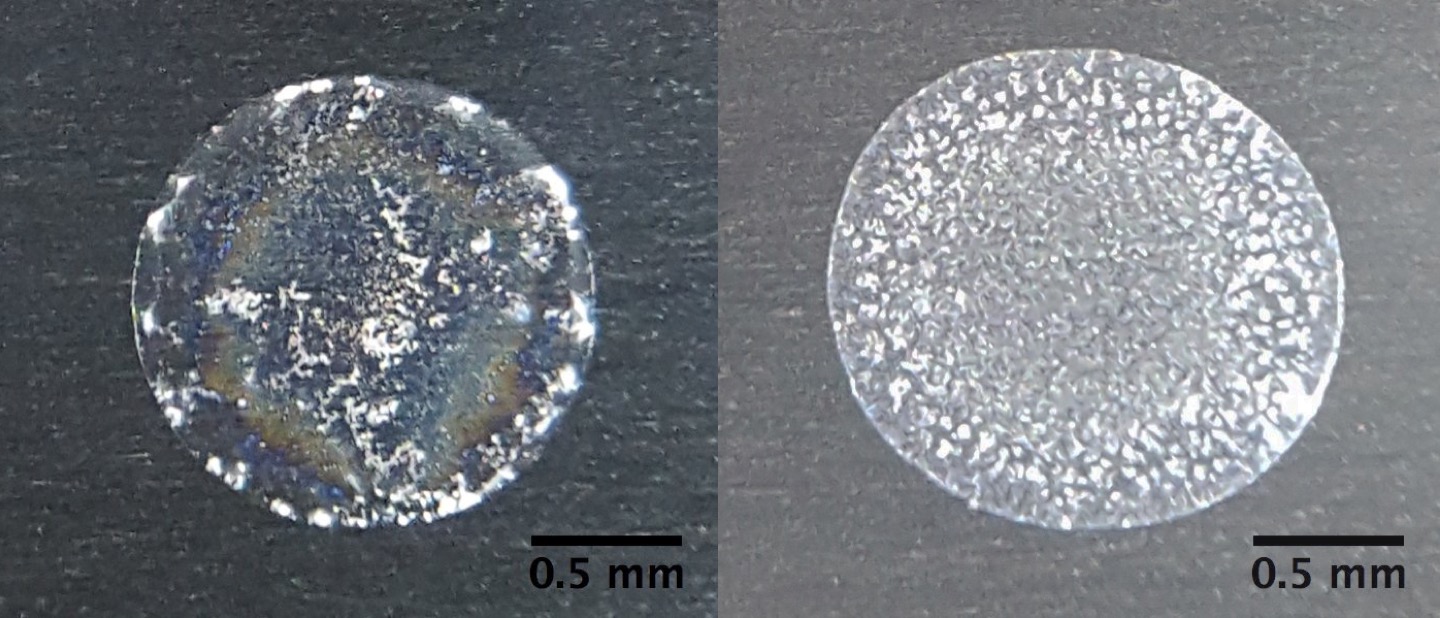
The physics of the so-called "coffee-ring effect" — how particles in liquid cause darkened areas to form at the perimeter of a spill — is helping scientists to quickly and cheaply identify the mineral contents of tap water, according to new research.
Residues left behind when tap water evaporates are "like fingerprints" for the water's properties and contents, Rebecca Lahr, an assistant professor in the chemistry department in Lyman Briggs College at Michigan State University, told the American Chemical Society (ACS) in a statement.
Compared to coffee rings, the patterns and whorls produced by minerals in tap water are highly intricate. And while you may not be able to detect the specific blend of beans that produced a coffee ring, Lahr and her colleagues are finding a wealth of information in the mineral traces from water droplets, she told the ACS. [Why Does My Water Taste Weird?]
Testing methods for tap water can be expensive and time-consuming, and the researchers wanted to find a way that would allow anyone, anywhere in the world, to rapidly and inexpensively identify certain signatures in drinking water, using the water's own residue patterns, Lahr said in a video.
Over time, recognizable patterns emerged for water quality, and the patterns that the researchers saw were consistent across tap-water samples gathered from the same communities across the southern Michigan area, the ACS reported.
These techniques could be effective ways to engage people in examining their own water, Lahr said in the statement. They also could be used to build a database of known residue patterns created by tap water under normal conditions that could help people quickly spot anomalies that may affect their water quality.
The findings were presented at the 254th National Meeting & Exposition of the American Chemical Society, held Aug. 20 to 24 in Washington, D.C.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Original article on Live Science.

Mindy Weisberger is a science journalist and author of "Rise of the Zombie Bugs: The Surprising Science of Parasitic Mind-Control" (Hopkins Press). She formerly edited for Scholastic and was a channel editor and senior writer for Live Science. She has reported on general science, covering climate change, paleontology, biology and space. Mindy studied film at Columbia University; prior to LS, she produced, wrote and directed media for the American Museum of Natural History in NYC. Her videos about dinosaurs, astrophysics, biodiversity and evolution appear in museums and science centers worldwide, earning awards such as the CINE Golden Eagle and the Communicator Award of Excellence. Her writing has also appeared in Scientific American, The Washington Post, How It Works Magazine and CNN.
 Live Science Plus
Live Science Plus