Lab-Grown 'Living' Bones Could Yield Customized Implants

For the first time, pieces of living bone have been grown from the cells of patients — in this case, miniature pigs — and sculpted to replace missing anatomical structures.
The custom-engineered bone was used to successfully repair a pig's lower jaw, one of the strongest and most complex jaws in the face, paving the way for bone repairs that could be carried out elsewhere in the body, the researchers said.
Bones often come in complex shapes, making it difficult to find matching natural replacements for them in patients suffering from injuries, diseases or birth defects. Although surgeons can replace missing bone with titanium, such artificial implants lack bone marrow, which plays many important roles in the body, such as generating red blood cells and immune cells. [The 9 Most Interesting Transplants]
Patients could receive donated bones, but doing so raises other issues, such as increasing the risk of tissue rejection. Alternatively, doctors can harvest bone from another part of a patient's body and carve it to fit where it needs to go, but damage at the harvest site is typically major and can lead to extreme pain.
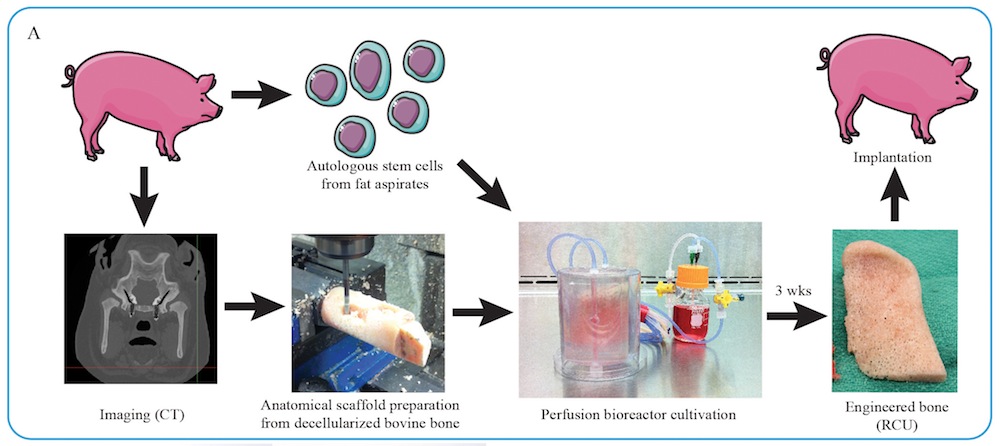
Instead, scientists now hope to grow living bone in the lab. The researchers focused on the ramus-condyle unit, the part of the lower jaw that meets the skull near the ear, and the main weight-bearing bone in the face. They experimented on Yucatán minipigs, because the animals' jaw anatomy and mechanics closely mimic those of humans.
The researchers first took chunks of cow thighbones and stripped them of all their cells using detergents and enzymes. They next carved these "decellularized" chunks into perfect anatomical fits for the ramus-condyle units that were surgically removed from the minipigs.
The scientists then seeded these bone scaffolds with stem cells derived from the fat of the minipigs that were to receive these grafts. The implants were then placed in "bioreactors" that supplied the stem cells with oxygen and nutrients.
Get the world’s most fascinating discoveries delivered straight to your inbox.
After three weeks, the stem cells developed into immature living bone. "The bone is formed by the recipient's own cells," study senior author Gordana Vunjak-Novakovic, a bioengineer at Columbia University, told Live Science.
But, if these living bone grafts ever make it to the clinic, they may be grown far away from where they are eventually implanted. To see how their grafts might fare under such conditions, Vunjak-Novakovic and her colleagues manufactured and implanted the grafts, "at two locations that were more than 1,200 miles (1,930 kilometers) apart, New York City and Baton Rouge (Louisiana)," Vunjak-Novakovic said. Fat cells were shipped from the pigs to the researchers, and the grafts were shipped in their bioreactors to the pigs.
Six months after implantation, these grafts successfully incorporated themselves into their host bodies and regenerated bone without any complications, while also helping the minipigs use their jaws again, the researchers said. Moreover, "unexpectedly, the lab-grown bone, when implanted, was gradually replaced by new bone formed by the body," Vunjak-Novakovic said. "This feature is what makes this implant your own bone that will become an integral part of the native bone." [7 Cool Uses of 3D Printing in Medicine]
Vunjak-Novakovic noted that the quality of the regenerated tissue exceeded that of previous approaches. Moreover, the scaffold they developed enabled bone formation without the use of expensive chemicals known as growth factors that other approaches typically rely on.
"This is a very exciting step forward in improving regenerative medicine options for patients with craniofacial defects, and we hope to start clinical trials within a few years," Vunjak-Novakovic said in a statement.
The clinical trials with living bone grafts would be conducted through Vunjak-Novakovic's company epiBone.
"Having a chance to work on innovative research that may be part of our future is intriguing, energizing, and really inspiring," said study lead author Sarindr Bhumiratana, a postdoctoral fellow at Columbia University, who is also the chief scientific officer at epiBone.
The scientists are now also experimenting with including a cartilage layer on their living bone grafts to more closely mimic natural bone. "Cartilage is a thin and resilient tissue that lines the ends of most of our bones, to enable frictionless motion," Vunjak-Novakovic said.
The scientists detailed their findings online June 15 in the journal Science Translational Medicine.
Original article on Live Science.