Cancer Tech: New Devices Could Speed Up Treatment
Treating cancer is sometimes a process of trial and error, because any given drug or drug combination does not work the same for all patients. Precious time can be lost while doctors seek the right chemicals to beat back a tumor.
Now, two research teams say they have found ways to speed up the process by allowing doctors to try multiple treatments at once: One is an implantable device, and the other is a special injection device.
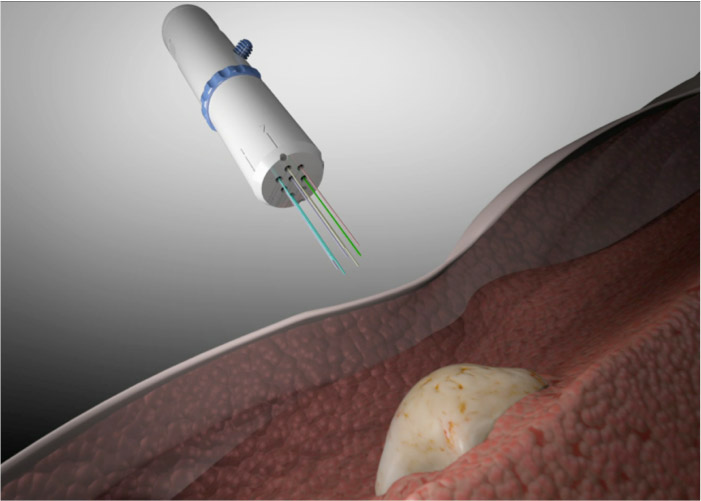
In Seattle, researchers at the Fred Hutchinson Cancer Research Center and the company Presage Biosciences designed a device called CIVO that includes up to eight needles arranged in an array. The device can be used to inject multiple drugs into tumors that are close to the surface of a person's skin.
First, the needles are loaded with drugs, pressed into the tumor and then withdrawn, with each needle leaving behind a columnlike trail of a drug that spans the full depth of the tumor.
Then, one to three days later, researchers can remove a piece of the tumor and examine the cells to see the effect of each drug — whether it killed the tumor cells, slowed their growth or had no effect. That analysis can tell doctors whether a certain drug or set of drugs will be more effective.
"Ordinarily, when I write a prescription, I have no way to know if the cancer is resistant" to the drug that's being prescribed, said Dr. James Olson, a pediatric oncologist at Fred Hutchinson and the senior author of the CIVO report, published today (April 22) in the journal Science Translational Medicine. [Top 10 Cancer-Fighting Foods]
With CIVO, doctors "can compare drug A to drug B," Olson said. The device could also be a boon to drug development, as it allows for controlled experiments that don't require flooding a patient's system with experimental chemotherapy drugs, he said.
Get the world’s most fascinating discoveries delivered straight to your inbox.
So far, the device has been tested on mice, 20 dogs and four human patients. The four human patients all had lymphomas, which are cancers of the lymph system, and had enlarged lymph nodes. The patients said they had very little pain with the injections, according to the report.
Meanwhile, researchers at MIT have built a cylindrical device the size of a rice grain that is riddled with microscopic tubes. Each tube can contain a different drug, and the device can carry up to 30 drugs, according to the researchers' report, also published today in Science Translational Medicine.
Unlike CIVO, the cylinder is designed to be implanted into the tumor, and then diffusion allows the drugs to move from the tubes into the surrounding cancerous tissue. A biopsy of the tumor is taken a day or two later — a doctor removes the cylinder and a small amount of the cancer tissue around it.
As with CIVO, the aim is to let doctors look at the cancerous tissue, to see which drugs worked better or which ones didn't work at all. "It's a way to predict whether the patient will respond to the drug or not," said Robert Langer, a professor of bioengineering and chemical engineering at MIT who is one of the senior authors on the report.
So far, the implant has been tested only in mice, so it will likely take longer than CIVO to get into clinical testing. But the implant offers a way to attack cancers that are deeper in the body and thus less accessible to injections. Langer said his team is putting together study proposals for clinical trials.
Olson said the ability to test out drugs using such devices could make chemotherapy more comfortable for patients because doctors will know early on whether certain drugs will work for a given patient. That would make it less likely that patients would have to endure ineffective chemo treatments — with all of their associated side effects — and would also save time in the process, Olson said.
Even knowing that no drug will help a patient could be a good thing, he said, because then doctors could make him or her comfortable, and the patient would avoid enduring the side effects of drugs that wouldn't end up treating the cancer.
"Some drugs make patients sick," he said. "It would be great if we could do nothing more than prevent that."
The Seattle researchers' work was funded by the National Institutes of Health and Presage Biosciences, and the MIT researchers' work was funded by the National Cancer Institute and Massachusetts-based biotech company Kibur Medical.
Follow Live Science @livescience, Facebook & Google+. Original article on Live Science.

 Live Science Plus
Live Science Plus





