FDA Approves Meningitis B Vaccine
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
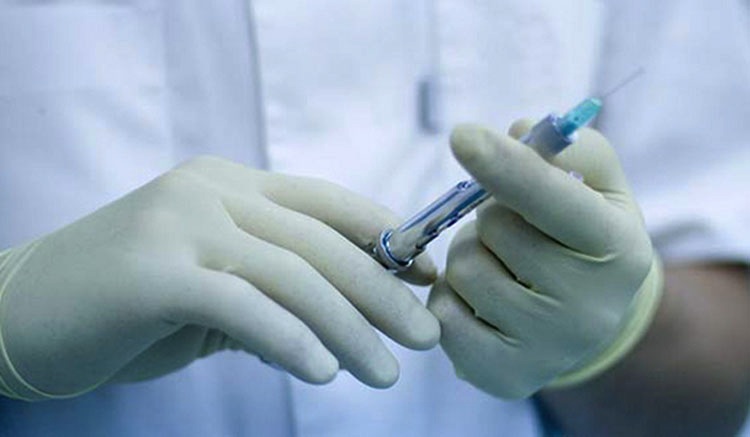
The first vaccine designed to prevent a common type of bacterial meningitis has been approved for use in the United States.
The U.S. Food and Drug Administration (FDA) announced today that it has approved Trumenba, which is made by a subsidiary of Pfizer called Wyeth Pharmaceuticals. The vaccine is intended to prevent a life-threatening disease caused by Neisseria meningitidis serogroup B. This type of bacteria has been blamed for recent meningitis outbreaks on college campuses, including an outbreak at Princeton University that was linked to the death of a young woman in Philadelphia.
"Recent outbreaks of serogroup B Meningococcal disease on a few college campuses have heightened concerns for this potentially deadly disease," Karen Midthun, director of the FDA's Center for Biologics Evaluation and Research, said in a statement. "The FDA's approval of Trumenba provides a safe and effective way to help prevent this disease in the United States." [5 Dangerous Vaccination Myths]
The bacteria N. meningitidis is a leading cause of bacterial meningitis, but until now, the vaccines approved for use in the United States covered only four of the five main serogroups, or types, of N. meningitidis: A, C, Y and W. These vaccines did not protect against serogroup B.
Meningococcal disease is caused by bacteria that infect the bloodstream and cause inflammation in the tissue that lines the brain and spinal cord. The bacteria is transmitted from person to person through respiratory and throat secretions, such as saliva, that are shared during close contact, kissing, coughing and sharing utensils. Symptoms include fever, headache, nausea, vomiting and a stiff neck.
Meningococcal disease seems to most commonly strike teens and young adults — especially those living in close quarters. That's why outbreaks are common on college campuses.
Last year, outbreaks of meningitis began at both Princeton University in New Jersey and the University of California, Santa Barbara. Both were caused by Neisseria meningitidis serogroup B. One student who became ill at Santa Barbara had to have both of his feet amputated. A student at Drexel University in Philadelphia died after becoming infected with the Princeton strain of the bacteria; before the onset of her illness, she had had close contact with students from Princeton, according to the New Jersey Department of Health.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Last year, the FDA allowed a different, still unapproved vaccine called Bexsero (made by the drug company Novartis) to be administered to help control outbreaks at Princeton and Santa Barbara. The newly approved drug, Trumenba, went through an accelerated approval process, FDA officials said. The agency said it reviewed the drug's safety and effectiveness in well under six months.
Follow Megan Gannon on Twitter and Google+. Follow us @livescience, Facebook & Google+. Original article on Live Science.

 Live Science Plus
Live Science Plus





