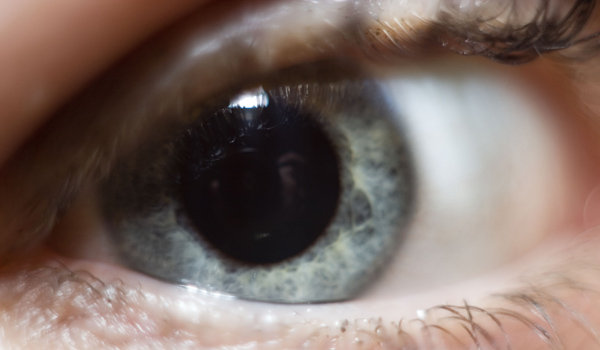
Cancer Drug Works to Treat Aging Eyes
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
The cancer drug Avastin works just as well at treating age-related macular degeneration as the Food and Drug Administration-approved drug Lucentis, according to a new study by the National Eye Institute.
Age-related macular degeneration is the No. 1 cause of blindness and vision loss in older people in the United States, and is caused by growth of abnormal blood vessels that leak fluid in the eyes. The fluid damages the macula, which is the central part of the retina responsible for central vision and perception of fine detail.
Avastin, developed by drug company Genentech, was originally approved by the FDA in 2004 to stop the growth of blood vessels that allow colon cancer tumors to develop and spread. Genentech later developed Lucentis, made from a similar protein as Avastin as a treatment to stop blood vessel growth in people with age-related macular degeneration .
However, before the FDA approved Lucentis in 2006, many ophthalmologists used Avastin off-label for their patients with age-related macular degeneration. Off-label use for drugs is legal, and doctors may choose to prescribe an off-label drug for a patient if it has a track record (even if not yet recognized by the FDA) for treating the condition.
The study shows that Avastin is safe for off-label use for treating age-related macular degeneration, and seems equally effective as Lucentis, researchers said.
The study will be published online May 1 in the New England Journal of Medicine.
Monthly dose or taken as needed?
Get the world’s most fascinating discoveries delivered straight to your inbox.
Doctors who use Avastin to treat age-related macular degeneration often use the drug primarily as needed, but there was no data to see if this sort of treatment regimen was as effective as Lucentis' monthly dosing schedule.
Therefore, researchers set out to compare Lucentis and Avastin in 2008 with the Comparison of AMD Treatments Trials, which included 1,185 patients with wet age-related macular degeneration , the advanced form of the disease. Forty-three clinical centers across the United States participated in the trial.
Patients were assigned to one of four treatment regimens for one year: Lucentis monthly, Avastin monthly, Lucentis primarily as needed or Avastin primarily as needed, according to the study.
Patients who received the drugs monthly were given an initial treatment and then a subsequent injection of the drug every 28 days. But patients in the "primarily as needed" groups were given an initial treatment and then examined every 28 days to see if they needed more treatment, as indicated by fluid in the retina, the study said. Patients who received the drugs primarily as needed had four to five fewer injections over the course of the year than those who received the monthly treatment.
Neither patients nor ophthalmologists knew which study drug they were receiving, the study said.
A year later, visual acuity was virtually identical (only one letter difference on an eye chart) between the patients who received the monthly dose of Avastin and the monthly dose of Lucentis, researchers found.
The patients who received the primarily as needed dosing had slightly poorer visual acuity at the end of the study (two letters less than those with the monthly dosing), but Lucentis and Avastin still performed equally, the study said.
Adverse events
The number of adverse events (where patients hospitalized) associated with Avastin was slightly higher (24 percent) than with Lucentis (19 percent), but researchers caution that there is no way to confirm that the treatments caused the adverse events.
Death, heart attack and stroke occurred at very low rates in the study, and occurred with similar frequency between the two drugs, researchers said.
Next, the researchers hope to follow the patients through a second year of treatment to gather information on long-term effects and safety of the drugs.
Pass it on: Cancer drug Avastin works just as well at treating age-related macular degeneration as the FDA-approved drug Lucentis.
- 5 Experts Answer: Whatâ??s the Best Way to Preserve My Eyesight?
- 3 Ways Technology Affects Your Eyes
- Macular Degeneration: Symptoms, Diagnosis & Treatments
Follow MyHealthNewsDaily staff writer Amanda Chan on Twitter @AmandaLChan.
 Live Science Plus
Live Science Plus