Chikungunya Vaccine Shows Promise
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
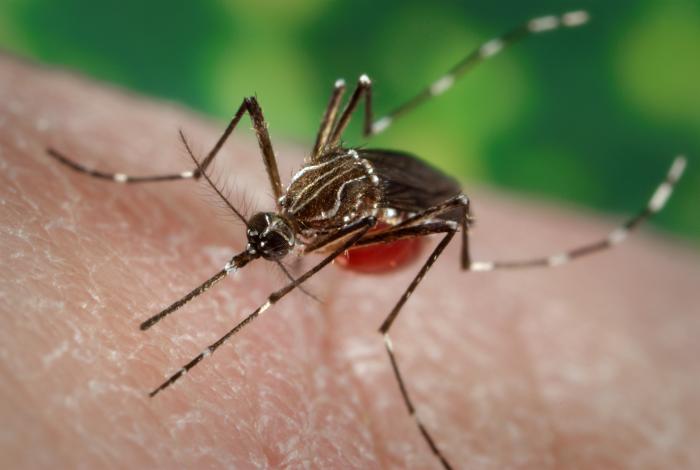
A new vaccine against chikungunya — a virus that showed up in the United States for the first time this year, and which can cause debilitating joint pain — shows promise in early tests in people, according to a new study.
In this small study, the vaccine appeared to be safe, and produced an immune response that researchers suspect would protect people against infection. Larger studies are now needed to confirm the results, the researchers said.
The chikungunya virus is spread by mosquitoes, and is native to regions in Africa and Southeast Asia, but last year the virus spread to the Americas for the first time, including the Caribbean. This year, four Americans have caught the virus while in the United States — the first cases of people catching the disease within this country — and another 580 caught the virus while traveling abroad, according to the Centers for Disease Control and Prevention.
Most people who are infected develop symptoms that include fever and painful arthritis. The disease is rarely fatal, but the pain can persist for months, the CDC says. There is currently no treatment for the disease, nor is there an approved vaccine to prevent it. [7 Devastating Infectious Diseases]
In the new study, researchers tested the experimental vaccine on 25 healthy people in Maryland, who were divided into three groups, with each group receiving a different dose of the vaccine. Participants were given three shots over a 20-week period.
No serious reactions to the vaccine were reported, but 10 participants experienced mild reactions, such as headache, nausea or a feeling of being unwell after the shot. All participants developed high levels of antibodies to fight the virus after the second vaccination.
The researchers cannot determine for certain whether the vaccine would protect people from becoming infected with chikungunya virus. But the participants in the study had antibody levels that were comparable to those seen in people who recovered from natural infection. "This observation gives us additional confidence that this vaccine would provide as much protection as natural infection," study researcher Dr. Julie Ledgerwood, of the National Institute of Allergy and Infectious Diseases, said in a statement.
Get the world’s most fascinating discoveries delivered straight to your inbox.
A previous study found that the vaccine protected monkeys from becoming infected with chikungunya virus.
The new study "represents an important step in vaccine development to combat this rapidly emerging pathogen," the researcher wrote in the Aug. 15 issue of the journal the Lancet.
The vaccine is made from viruslike particles that contain the outer shell of the virus, but do not have the genetic material the virus needs to make copies of itself, the researchers said.
The new vaccine "exhibits a range of properties that suggest it would be a good vaccine option," Ann Powers of the CDC wrote in an editorial accompanying the study.
However, Powers said that the development of vaccines for "orphan" diseases such as chikungunya, which affect a relatively small number of people, is challenging because "the market might not be large enough to justify the investment." The cost of vaccine development can range from $200 million to $500 million, she noted.
Still, "in view of the burden of chikungunya outbreaks, which have affected up to 63 percent of local populations in a matter of months, the continued development of this VLP vaccine candidate, along with other vaccine options, should be encouraged," Powers said.
Follow Rachael Rettner @RachaelRettner. Follow Live Science @livescience, Facebook & Google+. Original article on Live Science.

Rachael is a Live Science contributor, and was a former channel editor and senior writer for Live Science between 2010 and 2022. She has a master's degree in journalism from New York University's Science, Health and Environmental Reporting Program. She also holds a B.S. in molecular biology and an M.S. in biology from the University of California, San Diego. Her work has appeared in Scienceline, The Washington Post and Scientific American.
 Live Science Plus
Live Science Plus










