Earth’s Future: Scary Ozone Scenario Thwarted
If 193 nations hadn’t agreed in 1989 to ban the chemicals that eat up the Earth’s protective ozone layer, the world would have been a much different place later this century, with nearly two-thirds of the ozone layer gone and the ozone hole a permanent fixture over Antarctica, a new simulation shows.
Sunburns would occur in a matter of minutes and skin cancer-causing radiation would soar.
Ozone is the Earth's natural sunscreen, absorbing and blocking most of the incoming ultraviolet (UV) radiation from the sun and protecting life from the DNA-damaging rays.
The gas is naturally created and replenished by a photochemical reaction in the upper atmosphere where UV rays break oxygen molecules (O2) into individual atoms that then recombine into three-part molecules of ozone (O3).
As it is moved around the globe by upper level winds, ozone is slowly depleted by naturally occurring atmospheric gases. It is a system in natural balance.
But chlorofluorocarbons (CFCs) — invented in 1928 as refrigerants and as inert, or non-reacting, carriers for chemical sprays — upset that balance.
Researchers discovered in the 1970s and 1980s that while CFCs are inert at Earth's surface, they are quite reactive in the stratosphere (6 to 31 miles altitude, or 10 to 50 kilometers), where roughly 90 percent of the planet's ozone accumulates.
Get the world’s most fascinating discoveries delivered straight to your inbox.
UV radiation causes CFCs and similar bromine compounds in the stratosphere to break up into elemental chlorine and bromine that readily destroy ozone molecules. Worst of all, such ozone depleting substances can reside for several decades in the stratosphere before breaking down.
In the 1980s, ozone-depleting substances opened a wintertime "hole" over Antarctica, which served as the impetus for the 1989 Montreal Protocol, which banned CFCs.
The United States signed on to the original Montreal Protocol agreement, as did many other countries, including China, India, Iran and Brazil.
"The regulation pre-supposed that a lack of action would lead to severe ozone depletion, with consequent severe increases of solar UV radiation levels at the Earth’s surface," said Paul Newman of NASA's Goddard Space Flight Center in Greenbelt, Md.
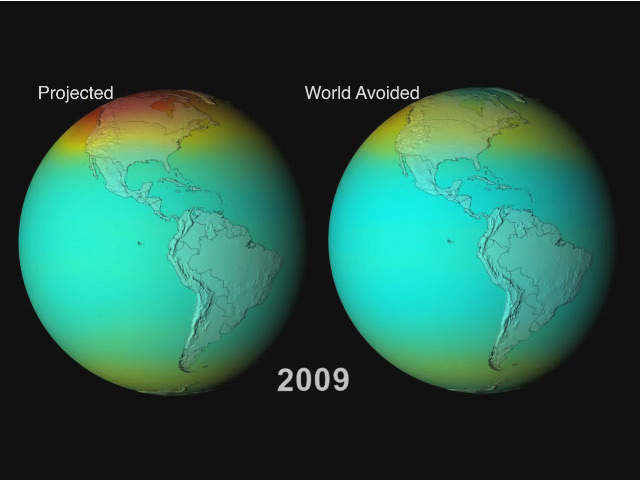
Goddard and his colleagues used computer simulations to show "what might have been" if Montreal Protocol had never been put in place.
The team used a computer model that simulates the circulation of the Earth's atmosphere and takes into account how changing levels of ozone affect that circulation. They increased the emission of CFCs and similar compounds by 3 percent per year, a rate about half the growth rate for the early 1970s. Then they let the simulated world evolve from 1975 to 2065.
By the simulated year 2020, 17 percent of all ozone is depleted globally, as assessed by a drop in Dobson Units (DU), the unit of measurement used to quantify a given concentration of ozone. An ozone hole starts to form each year over the Arctic, which was once a place of prodigious ozone levels.
By 2040, in the scenario that is not actually happening, global ozone concentrations fall below 220 DU, the same levels that currently comprise the "hole" over Antarctica. (In 1974, globally averaged ozone was 315 DU.) The UV index in mid-latitude cities reaches 15 around noon on a clear summer day (a UV index of 10 is considered extreme today), giving a perceptible sunburn in about 10 minutes. Over Antarctica, the ozone hole becomes a year-round fixture.
In the 2050s, ozone levels in the stratosphere over the tropics collapse to near zero in a process similar to the one that creates the Antarctic ozone hole.
By the end of the model run in 2065, global ozone drops to 110 DU, a 67 percent drop from the 1970s. Year-round polar values hover between 50 and 100 DU (down from 300 to 500 in 1960). The intensity of UV radiation at Earth's surface doubles; at certain shorter wavelengths, intensity rises by as much as 10,000 times. Skin cancer-causing radiation soars.
"We simulated a world avoided," said Newman, "and it's a world we should be glad we avoided."
Some of the results of the simulation, detailed online in the journal Atmospheric Chemistry and Physics, were unexpected, even to the scientists on the team.
"Our 'world avoided' calculation goes a little beyond what I thought would happen," said Goddard scientist and study team member Richard Stolarski. "The quantities may not be absolutely correct, but the basic results clearly indicate what could have happened to the atmosphere. And models sometimes show you something you weren't expecting, like the precipitous drop in the tropics."
The real world of CFC regulation has been somewhat kinder. Production of ozone-depleting substances was mostly halted about 15 years ago, though their abundance is only beginning to decline because the chemicals can reside in the atmosphere for 50 to 100 years. The peak abundance of CFCs in the atmosphere occurred around 2000, and has decreased by roughly 4 percent to date.
Stratospheric ozone has been depleted by 5 to 6 percent at middle latitudes, but has somewhat rebounded in recent years. The largest recorded Antarctic ozone hole was recorded in 2006.
- Top 10 Ways to Destroy Earth
- Man and Nature Combine to Create Ozone Holes
- Is Ozone Good or Bad?