Weird 'gut-eye axis' links the retina and intestines, and may help explain glaucoma
A type of immune cell that travels from the gut to the eyeballs may help to explain why some people with glaucoma continue to lose their vision after treatment.
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
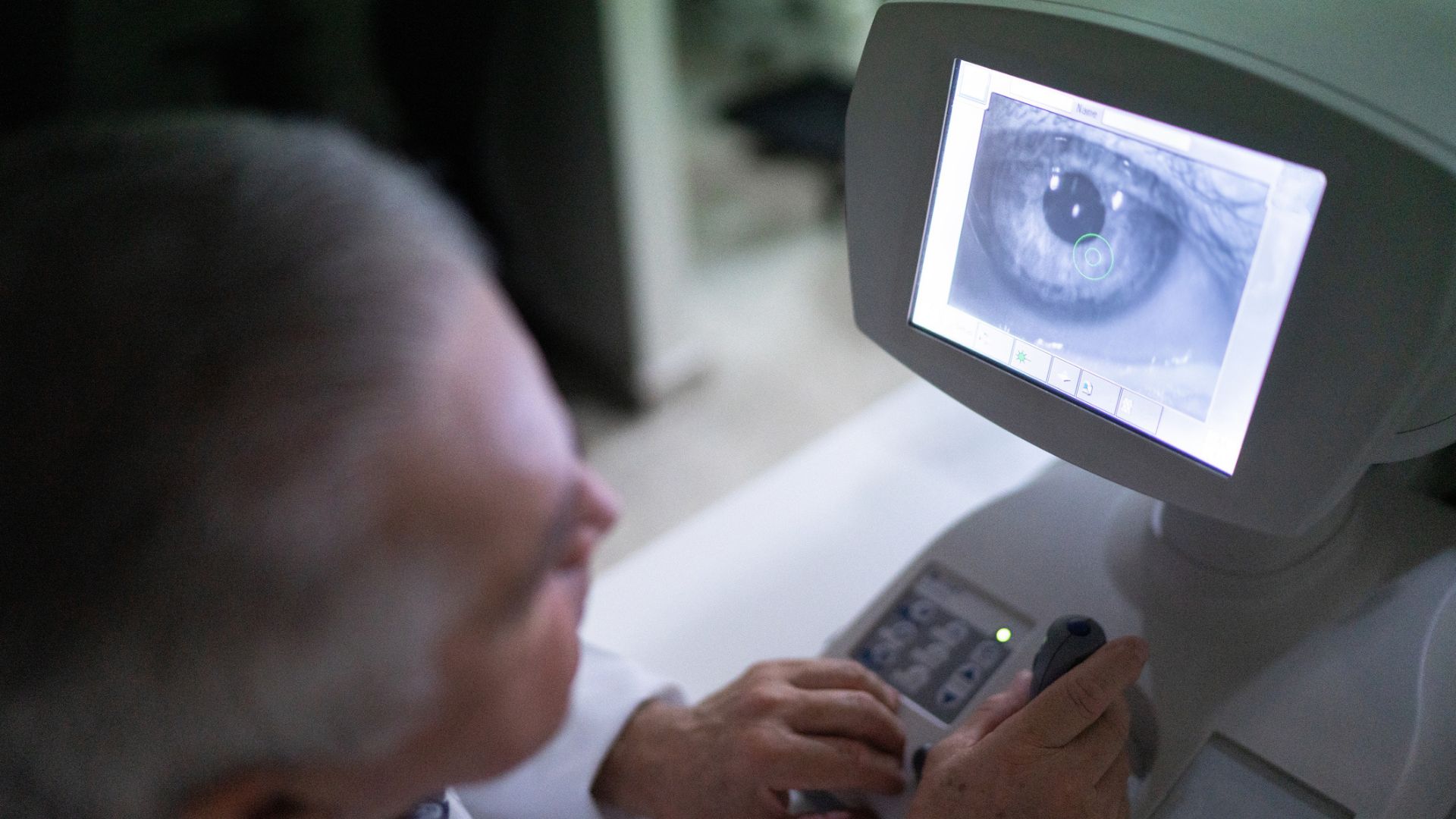
Glaucoma, an eye disease that gradually causes blindness by damaging the optic nerve, is often triggered by excess pressure from fluid in the eye — but some people still lose vision even after that pressure is relieved.
Now, new research points to a surprising reason why: A group of immune cells from the gut can gain the ability to infiltrate the retina, the light-sensitive tissue in the back of the eye, wreaking havoc. These cells — disease fighters known as "helper T cells" that carry a protein called beta 7 — do not normally have the ability to cross the optic nerve into the eye, but something about the early stages of glaucoma seems to trigger a bizarre activation pattern that ends up altering the T cells and worsening disease progression.
"In our study, we observed that 'gut education' potentially leads to a fundamental reprogramming of T cells in the peripheral blood of glaucoma mice," said study co-leader Fang Lu, a researcher at the Sichuan Academy of Medical Sciences and Sichuan's People's Hospital in China.
Previous studies had shown that, in both mice and humans, glaucoma is marked by an infiltration of T cells into the retina. When these specific T cells are transferred into a healthy mouse retina, they cause glaucoma-like damage. The study's researchers also previously found that these T cells express a receptor that lets them travel from the gut to the retina. Once in the eye, these "gut-licensed" cells can grab onto a molecule in the lining of the retina in order to pass into the tissue.
Related: Gut bacteria may 'talk' to the brain, mouse study suggests
"Based on these findings, we hypothesized that there might be a connection between gut-exposed T cells, their migration to the retina, and the progression of glaucoma," Lu told Live Science.
In the new study, published in the journal Science Translational Medicine Wednesday (Aug. 2), the team found that people with glaucoma have elevated levels of these gut-licensed T cells in their blood. They discovered this by analyzing the blood of nearly 520 glaucoma patients and about 190 patients without glaucoma. The higher the level of gut-licensed cells in a person's bloodstream, the greater their glaucoma damage.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Using a mouse model of glaucoma, the team revealed that these cells use their licenses to visit the gut, where they're genetically "reprogrammed" to switch on different genes than normal. The cells then hightail it to the retina, where they directly contribute to damage. It's the reprogramming that gives the cells what Lu called "a remarkable ability to target the retina."
The researchers identified a protein within the retina that the gut-exposed T cells can bind to, allowing them to sneak into the eye tissue; normal T cells cannot latch onto this protein. When the researchers blocked this protein and T cell interaction, they saw significantly less glaucoma damage.
The researchers aren't yet sure what prompts these T cells to go rogue. But they did find that, when the T cells are exposed to elevated eye pressure, they migrate to the gut in mice. Thus, the local stress of increased eye pressure might trigger an inflammatory spiral in the body, which pushes the T cells into a dangerous activation pattern.
However, exactly what happens in the gut to reprogram these cells remains an open question, Lu said.
If the mechanisms uncovered in the mouse study hold true for humans, it may open up new avenues for treating glaucoma, Lu said. The team found that vedolizumab, a medication that treats inflammatory bowel disease by suppressing gut inflammation, prevented retinal damage in mice with increased eye pressure. Further research and clinical studies will be needed to see if that treatment holds any promise for glaucoma patients.
"Our next steps involve delving deeper into the role of the gut environment in modulating T cells," Lu said.

Stephanie Pappas is a contributing writer for Live Science, covering topics ranging from geoscience to archaeology to the human brain and behavior. She was previously a senior writer for Live Science but is now a freelancer based in Denver, Colorado, and regularly contributes to Scientific American and The Monitor, the monthly magazine of the American Psychological Association. Stephanie received a bachelor's degree in psychology from the University of South Carolina and a graduate certificate in science communication from the University of California, Santa Cruz.
 Live Science Plus
Live Science Plus