This Behind the Scenes article was provided to LiveScience in partnership with the National Science Foundation.
Cells in the human body live in amazingly complex three-dimensional environments that are crucial for their proper function. The lung, for example consists of layers of different kinds of cells that work together to exchange oxygen and carbon dioxide between the air and the blood.
The way these cells work together, and the chemicals that they express to communicate with one another, change when they live on a flat, two-dimensional surface.
Given these differences in cell behavior and expression, it's intriguing that the standard for testing new drugs and chemicals are tests that use cells grown in flat-bottomed Petri dishes.
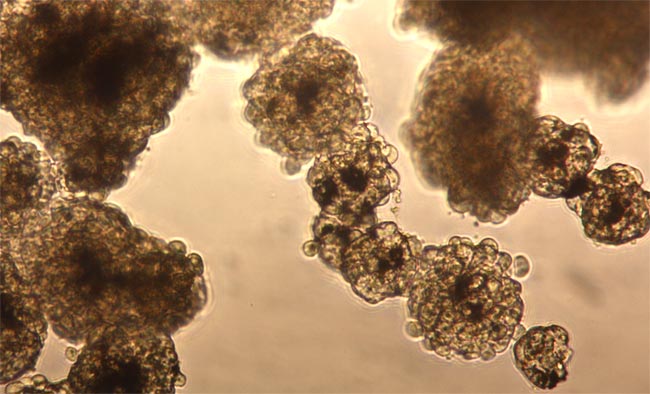
In an effort to more accurately mimic the effect of drugs or toxic chemicals on real living tissue, scientists from Rice University and the University of Texas' M.D. Anderson Cancer Center in Houston have developed a new laboratory technique that uses magnetic levitation to grow cells in three-dimensional shapes. Compared with cell cultures grown on flat surfaces, these 3-D cell cultures form tissues that more closely resemble those inside the body. The technique has the potential to drastically reduce the cost of developing new drugs, as well as to reduce the use of animals when testing the safety of manufactured chemicals. The team's results were published in March, 2010, in Nature Nanotechnology.
"There's a big push right now to find ways to grow cells in 3-D because the body is 3-D, and cultures that more closely resemble native tissue are expected to provide better results for pre-clinical drug tests," said study co-author Tom Killian, associate professor of physics at Rice. "If you could improve the accuracy of early drug screenings by just 10 percent, it's estimated you could save as much as $100 million per drug."
The new technique is an example of the innovation that can result when experts come together from disparate fields. Killian uses magnetic fields to trap and manipulate atoms that have been cooled to near absolute zero. He had been working on a new project with Rice bioengineer Robert Raphael on methods to use magnetic fields to probe cellular membranes.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Killian's friend, Glauco Souza, who was then studying with the center's professors Wadih Arap and Renata Pasqualini, mentioned one day that he was developing a gel that could load cells with magnetic nanoparticles.
"We wondered if we might be able to use magnetic fields to levitate the treated cells off the bottom of the petri dish, allowing them to grow in 3D," said Souza, who left M.D. Anderson in 2009 to co-found Nano3D Biosciences, a startup that subsequently licensed the technology from Rice and M.D. Anderson.
"When we tried it," Killian said, "we were shocked by how robustly the cells grew and how they displayed tissue shapes that resembled real tissue."
The 3-D technique is simple, fast, and requires no special equipment. These are big advantages compared to other technologies that have attempted to take cell culturing into the third dimension.
Souza said Nano3D Biosciences is conducting additional tests, and he is hopeful they will show magnetic levitation is as good, if not better, than longstanding techniques for growing 3-D cell cultures with scaffolds.
Nano3D Biosciences also has a grant from the National Science Foundation to use its technique to grow a layered model of lung tissue that can be used to test the toxicity of airborne chemicals.
Co-authors on the Nature Nanotechnology paper include Robert Raphael, Daniel Stark, Jeyarama Ananta and Thomas Killian of Rice; Glauco Souza and Carly Levin of Nano3D Biosciences; and Jennifer Molina, Michael Ozawa, Lawrence Bronk, Jami Mandelin, Maria-Magdalena Georgescu, James Bankson, Juri Gelovani, Wadih Arap and Renata Pasqualini, all of M.D. Anderson.
The research was funded by the National Science Foundation, M.D. Anderson's Odyssey Scholar Program, the Department of Defense's Breast Cancer Research Program, the David and Lucille Packard Foundation, the Gillson-Longenbaugh Foundation, the Marcus Foundation, AngelWorks, the National Institutes of Health and the National Cancer Institute.
- Top 10 Technologies That Will Transform Your Life
- New Device Prints Human Tissue
- 10 Profound Innovations Ahead
Editor's Note: This research was supported by the National Science Foundation (NSF), the federal agency charged with funding basic research and education across all fields of science and engineering. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author and do not necessarily reflect the views of the National Science Foundation. See the Behind the Scenes Archive.
