New Contact Lenses Pack Vitamin E, Could Replace Eye Drops
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
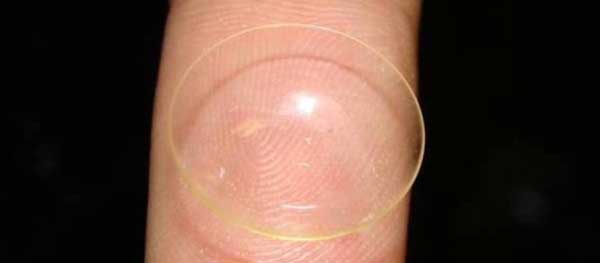
A new contact lens loaded with vitamin E could do away with eye drops.
The lenses could be used to deliver eye drops for glaucoma and cataracts, as well as less serious problems such as dry eyes, inflammation, and bacterial infections.
The problem with eye drops is that only a small amount (1 to 5 percent) of the drug actually gets to the cornea – a clear film covering the iris – the place it needs to go to do its job, said Anuj Chauhan, professor of chemical engineering at the University of Florida.
For eye problems like glaucoma, which can require several different eye-drop drugs taken two to three times daily, switching out lenses can be cumbersome.
Using contact lenses to more effectively deliver medicine to eyes isn’t a new concept. However, with today’s drug-loaded lenses “what happens is the drug comes out very very quickly, in about one hour or so,” Chauhan told TechNewsDaily.
The new medicated lenses solve this problem by using vitamin E to construct a chemical wall that prevents other medicines from just pouring out into the eye all at once.
Here's how it works: The vitamin forms barriers, what the researchers refer to as “nanobricks,” that the drug must maneuver around before entering the eye.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Vitamin E is water-hating, or "hydrophobic," but the drugs for many eye diseases are water-loving, so the drug must move around the vitamin bricks instead of moving through them. This creates a longer route to the eye.
Chauhan compared the vitamin E barrier to “buildings and traffic jams in Manhattan,” which make a trip from one end of the island to the other a long haul.
According to Chauhan, adding increasing amounts of vitamin E to a conventional contact lens extends the release of medicine from hours to days.
In experiments with lab animals, the researchers showed that the vitamin E-loaded lenses released drugs up to 100 times longer than commercial lenses.
Before the new medicated contact lenses can be sold in drug stores, they will have to go through human clinical trials, a process that will take at least five years, Chauhan said.
Chauhan and his colleagues presented their research at the 239th National Meeting of the American Chemical Society (ACS) being held in San Francisco this week.
 Live Science Plus
Live Science Plus