The Man Behind the Technology of "CSI"
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
This ScienceLives article was provided to LiveScience in partnership with the National Science Foundation.
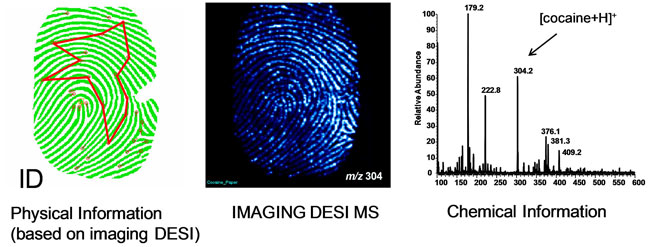
R. Graham Cooks, Purdue University's Henry Bohn Hass Distinguished Professor of Analytical Chemistry, has made mass spectrometry appeal to mass audiences with technology featured on the hit series "CSI." Mass spectrometry turns molecules into ions so their mass can be analyzed, and traditionally requires chemical separations, manipulations of samples and containment in a vacuum chamber. Cooks developed a desorption electrospray ionization technique, called DESI, that performs the ionization step in the air or directly on surfaces, making it much faster and more portable. Cooks and his research group have applied the DESI technology to societal issues ranging from the safety of food products to security at the airport. The team also has been working to reduce the size of the equipment to create a miniature mass spectrometer, which has been likened to the Star Trek "tricorder," a handheld sensing system used by the characters to analyze the chemical components of alien worlds. Cooks pioneered the use of DESI for detecting explosives residues on airport luggage, but it also has been used to detect the contaminant melamine in powdered milk and pathogens such as E. coli and Salmonella on produce. Because it was used to glean more than just identity from a fingerprint, the technology was featured in episodes of the hit shows "CSI" and "CSI: Miami." A paper illustrating the ability to detect trace amounts of explosives, drugs or other materials left behind in fingerprints, and to distinguish between overlapping fingerprints left by different individuals, was published in the journal Science in 2008. The technology is featured in an extensive press release about the work and a video interview. Learn more about Cooks and his work at his website, and below as he answers the ScienceLives 10 Questions.
Name: Graham Cooks Age: 68 Institution: Purdue University Field of Study: Analytical Chemistry
What inspired you to choose this field of study? My undergrad final exam as a math major coupled with a super-enthusiastic professor in chemistry (Frank Warren) who was totally involved in research.
What is the best piece of advice you ever received? "If you worry about tenure you don't deserve it." It applies much more generally.
What was your first scientific experiment as a child? Asking for and receiving on my 12th birthday two thermometers — medical and outdoor — and then taking data (I've been asking for instruments ever since, from my group, from NSF, etc).
What is your favorite thing about being a researcher? The excitement of the chase — the primitive hunting instinct very much alive even in us non-hunters.
Get the world’s most fascinating discoveries delivered straight to your inbox.
What is the most important characteristic a researcher must demonstrate in order to be an effective researcher? Uneven temperament — the agony and the ecstasy must be close to being unbearable.
What are the societal benefits of your research? I made a significant contribution to tandem mass spectrometry, widely used in the pharmaceutical industry, and many other fields. More recently, I've helped develop a number of promising methods for making ions for mass spectrometry so that explosives can be detected accurately in almost real time and a method for taking chemical fingerprints, which was seen on two shows: "CSI: Miami" and "CSI"-the-real-thing. This is moving towards medical diagnostics, which would be fabulous.
Who has had the most influence on your thinking as a researcher? My half dozen friends — all scientists, all passionate.
What about your field or being a researcher do you think would surprise people the most? That our chemistry does not involve solutions, or mixing or physical labor; it's chemistry in instruments that are really just computers with great electronics and real matter in tiny amounts. More students would take chemistry if they knew how much "technology" there is in analytical chemistry — like computer science caught in the act of mating with electrical engineering (also better known and CS + EE).
If you could only rescue one thing from your burning office or lab, what would it be? The handwritten text of a 1973 book, "Metastable Ions," which was so easy to write because one only wrote things once in those days.
What music do you play most often in your lab or car? Classical — whatever NPR has chosen that day.
 Live Science Plus
Live Science Plus










