Scientists Create Tiniest Blood Vessels
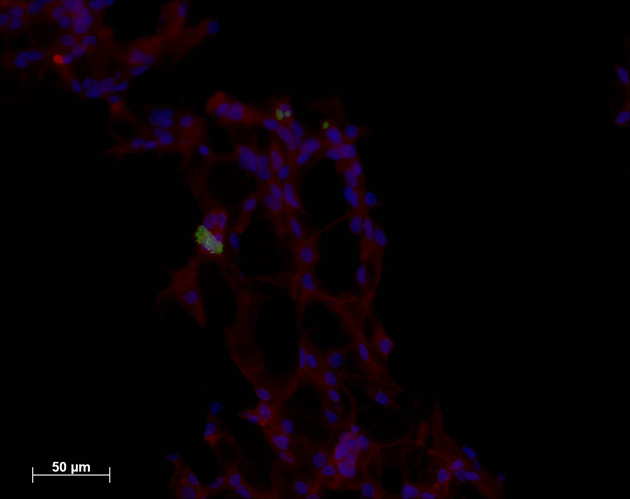
A gooey concoction of a biopolymer and two types of cells that, when finished, could pass as Dracula's potluck Jell-O mold might someday let surgeons replace the body's smallest blood vessels.
Tiny blood vessels extend the reach of veins and arteries, delivering oxygen to most of the body's tissues. When these smaller vessels fail, the tissues they support fail with them. Such damage is typical in advanced diabetes, for example, and is the reason diabetics sometimes must have limbs amputated.
Although surgeons have for years successfully transplanted large blood vessels, it has been too difficult to replace the minuscule venule, arteriole, and—the smallest of them all—capillary vessels, which are just 5 to 20 micrometers across. That's just too small to transplant piecemeal.
The solution
The solution, says Erin Lavik, an assistant professor of biomedical engineering at Yale University, may be to transplant entire networks of these diminutive vessels.
Other scientists also have developed transplantable blood-vessel networks, but those transplants haven't stuck. In fact, they have withered without linking to the body's vein-and-artery highways. Lavik's group hoped, however, that if engineered correctly their vascular side streets would spontaneously connect to the main routes that run to and from the heart.
The key is combining these two types of cells: endothelial cells, which are flat cells that line blood vessels and the heart, and neural stem cells, the brain's building blocks. Although scientists have suspected that these kinds of cells interact in the body, Lavik says, she and one of her colleagues—Yale School of Medicine pathology professor Joseph Madri—decided to introduce the cells to each other in a Petri dish.
Get the world’s most fascinating discoveries delivered straight to your inbox.
'This is wild'
Surprisingly, the endothelial cells, which usually grew just as a layer in the dish, began to form tubular structures.
"This is wild. This is not normally seen," Lavik told LiveScience. "Even if you remove the neural progenitor cells, those tubes are stable. They're going through some process where they stop just being cells and start forming tubes."
These self-assembling tubes were a giant step toward building an implantable network, Lavik said, but they were still just a tangle of impossibly small vessels piled limply in a lab dish. That's where Lavik's expertise in engineering polymers came in handy.
The scientists constructed a gelatinous scaffold from a hydrogel—a water-based jelly—that was riddled with tiny channels. Then they sprinkled the sponge-like scaffold with the vessel-building endothelial cells and neural stem cells. As they had in a dish, the endothelial cells formed tubes. But this time the tubes followed the scaffold's channels, forming a network of tiny blood vessels.
Testing in mice
When the scientists implanted these gelatinous scaffolds into little pockets just under the skin of lab mice, and then removed them as long as six weeks later, they found that not only had the new vessels survived, they had begun to connect to the critters' own larger (for a mouse) blood vessels.
If left in place long enough, Lavik says, the watery scaffolds would erode, leaving behind just the new network of blood vessels.
"Hopefully, if these guys actually form new tissue," she says," ultimately if you were to implant this you'd be left with tissue and no polymer in the long term."
The research was described earlier this year in the journal of the Proceedings of the National Academy of Sciences.
- Body Quiz: The Parts List
- Body Quiz: How the Parts Fit
- Body Quiz: What the Parts Do
- Synthetic Blood Vessels Not Such a Stretch
- Skin Stem Cells Made into Bone and Muscle
- Great Inventions: What do You Know?