Speedy TB Treatment Could Combat Drug Resistance
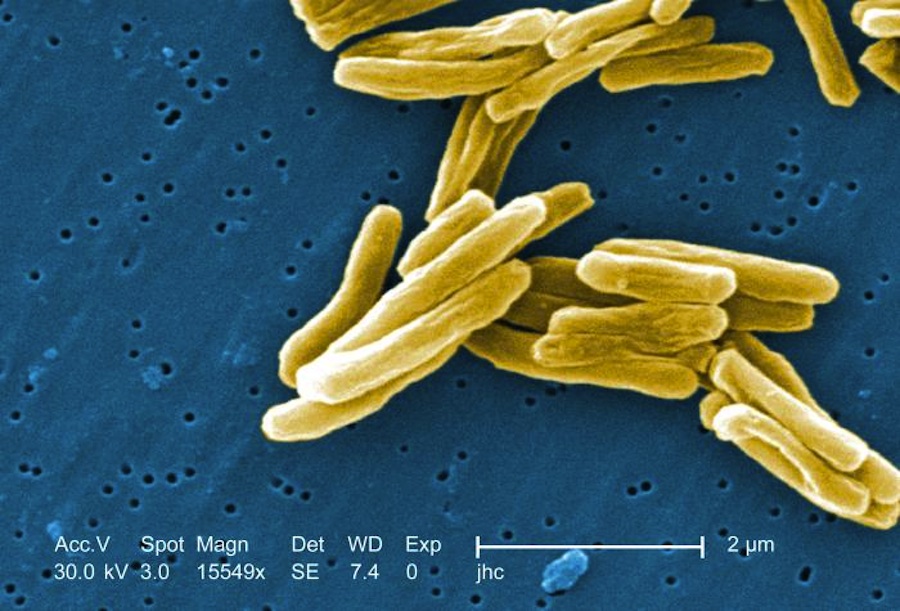
Tuberculosis in mice can be cured much faster than normal by simply tweaking the standard regimen of antibiotics, new research shows.
Scientists reduced treatment time in mice by up to 75 percent; they did so by optimizing the combinations and doses of the standard drugs. The finding may lead to a markedly shorter course of treatment for tuberculosis in humans and may reduce the risk of the infection becoming resistant to the antibiotics.
Treating tuberculosis in humans can be long and onerous; the infected person must take a regimen of antibiotics for eight months or more. As a result of this lengthy treatment, patients often fail to complete the full course of medication; this is particularly true in poor countries, where people may not have easy access to medical care. Failing to complete the course of antibiotics can lead to disease relapse, further spread of the illness and drug resistance. [27 Devastating Infectious Diseases]
The newly optimized combinations of antibiotics could reduce the spread of even the most virulent strains of tuberculosis, the researchers said. Their findings were published today (Jan. 24) in the journal Nature Communications.
Tuberculosis, or TB, is a leading cause of death worldwide, with more than 10.4 million cases and 1.8 million deaths in 2015, according to the World Health Organization (WHO). The disease is caused by the Mycobacterium tuberculosis bacterium. TB is an airborne disease spread by coughing, sneezing or any exchange of saliva.
Most worrisome to infectious-disease experts is the rise of multi-drug-resistant and extensively drug-resistant TB, against which few if any drugs are effective. There were more than 500,000 such cases in 2015, according WHO, mostly in China, India and Russia.
In the United States, 10 drugs are approved to treat TB. And the first line of defense is a set of four antibiotics: isoniazid, rifampin, ethambutol and pyrazinamide. These are usually given together for eight weeks, often followed by just isoniazid and rifampin for the next 16 to 24 weeks. [6 Superbugs to Watch Out For]
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
This standard regimen evolved from the 1950s to the present by a process of adding or replacing drugs one by one, rather than by a systematic search to identify the most highly synergistic drug combinations, explained the research team in the new study.
Given the literally billions of possible combinations of drugs and doses, the team set out to find which combination could be most effective at killing M. tuberculosis. The researchers were led by Dr. Marcus Horwitz, a professor of medicine at the University of California, Los Angeles (UCLA) David Geffen School of Medicine. The team used a special drug-screening technique developed at UCLA to rapidly identify the most promising drug combinations, and then tested them in a cell culture. That work was reported in March 2016.
This approach allowed the scientists to rank more than 1,000 three- and four-drug combinations in order of their potency in killing M. tuberculosis, Horwitz said.
In the new study, the team tested the most promising combinations on mice that were infected with TB. Two regimens stood out: One had four common and inexpensive drugs (clofazimine, ethambutol, prothionamide and pyrazinamide) and cured the mice in 12 weeks. The other had a similar set of four drugs but with a less-common, more-expensive drug (bedaquiline) replacing prothionamide. It cured the mice in only four weeks, a 75 percent reduction in time compared to the standard treatment. [7 Bizarre Drug Side Effects]
Dr. Daniel Clemens, an adjunct professor of medicine at the Geffen School of Medicine and a co-first author on the study, said he is guardedly optimistic that the regimens could work in humans.
"Showing markedly greater efficacy than the standard regimen in the mouse model makes our regimens very promising for further study in humans, but does not guarantee that they will be more effective in clinical trials," Clemens told Live Science.
"There are differences between TB in mouse and human that could cause our mouse model to overestimate the clinical efficacy of our regimens," he said. "On the other hand, the mouse model might underestimate the efficacy… [because] whereas a few residual bacteria in a mouse can cause relapse, the stronger human immune response may control a small number of bacteria and prevent relapse."
Clemens said his team found other drug combinations in the cell-culture studies that hold potential as "universal regimens for treatment of multi-drug-resistant TB" and that the researchers hope to test in mice and ultimately in humans.
Follow Christopher Wanjek @wanjek for daily tweets on health and science with a humorous edge. Wanjek is the author of "Food at Work" and "Bad Medicine." His column, Bad Medicine, appears regularly on Live Science.

Christopher Wanjek is a Live Science contributor and a health and science writer. He is the author of three science books: Spacefarers (2020), Food at Work (2005) and Bad Medicine (2003). His "Food at Work" book and project, concerning workers' health, safety and productivity, was commissioned by the U.N.'s International Labor Organization. For Live Science, Christopher covers public health, nutrition and biology, and he has written extensively for The Washington Post and Sky & Telescope among others, as well as for the NASA Goddard Space Flight Center, where he was a senior writer. Christopher holds a Master of Health degree from Harvard School of Public Health and a degree in journalism from Temple University.