HPV Vaccine Safe, But May Raise Risk of Fainting & Infections
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
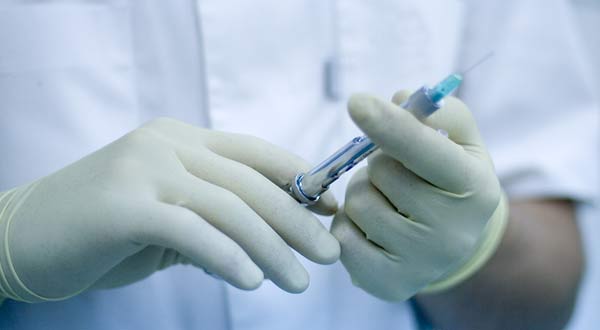
The human papillomavirus (HPV) vaccine is generally safe, but may increase the risk of fainting and skin infections shortly after vaccination, a new study finds.
The study included nearly 200,000 girls who received at least one dose of the quadrivalent HPV vaccine, known as Gardasil, which is marketed by Merck & Co. and protects against four strains of HPV.
Researchers found that fainting was six times more likely to occur on the day of vaccination, compared with a period many months after vaccination — there were 24 cases of fainting per 1,000 people on the day of vaccination, compared with an average of four cases per 1,000 people during a time period months after vaccination.
And skin infections were nearly twice as likely to occur within two weeks of vaccination compared with many months after vaccination. There were 3.5 cases of skin infections per 1,000 people during the two weeks after vaccination, compared with 2.2 cases per 1,000 people during the comparison time period, the researchers said.
Because these side effects were somewhat expected, and the study did not find any new safety concerns, the findings "support the general safety of routine vaccination," the researchers said.
HPV viruses are sexually transmitted viruses that usually cause no symptoms, but persistent infections can lead to cervical cancer. Gardasil was approved in 2006, after studies showed it was safe for use in females ages 9 to 26. But because studies conducted before a vaccine's approval are usually too small to detect rare side effects, researchers have continued to monitor the safety of the HPV vaccine.
In the new study, Nicola Klein, of Kaiser Permanente Vaccine Study Center in Oakland, Calif., and colleagues analyzed information from about 189,600 girls and young women, who received a total of 350,000 doses of the HPV vaccine between 2006 and 2008. The researchers calculated the annual rate of each side effect they observed.
Get the world’s most fascinating discoveries delivered straight to your inbox.
The researchers determined how likely participants were to be hospitalized or visit the emergency room for certain conditions in the 60 days after the vaccination, compared with a period many months after vaccination.
Injections in general are known to be linked with fainting, and so this result "is not unexpected," the researchers said.
There is evidence to suggest some of the skin infections seen in the study were actually reactions at the injection site, but the researchers did not have enough information to confirm this.
Unlike some earlier studies, the new study did not find an increased risk of blood clots linked with the vaccine. The researchers made sure to rule out side effects that were due to conditions the patients already had.
Ongoing studies of HPV are still needed to examine the risk of side effects, the researchers said. They noted that future studies should attempt to rule out effects that could be caused by pre-existing conditions.
The study was funded by Merck, and is published today (Oct. 1) in the journal Archives of Pediatrics & Adolescent Medicine.
Pass it on: The HPV vaccine is linked to an increased risk of fainting and skin infections.
This story was provided by MyHealthNewsDaily, a sister site to LiveScience. Follow Rachael Rettner on Twitter @RachaelRettner, or MyHealthNewsDaily @MyHealth_MHND. We're also on Facebook & Google+.

Rachael is a Live Science contributor, and was a former channel editor and senior writer for Live Science between 2010 and 2022. She has a master's degree in journalism from New York University's Science, Health and Environmental Reporting Program. She also holds a B.S. in molecular biology and an M.S. in biology from the University of California, San Diego. Her work has appeared in Scienceline, The Washington Post and Scientific American.
 Live Science Plus
Live Science Plus










