Rare 'super-diamonds' may already exist on other planets, and could be made on Earth, study hints
A simulated form of carbon called BC8, or 'super-diamond', could be 30% tougher than normal diamonds, but synthesizing it on Earth won't be easy.

Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
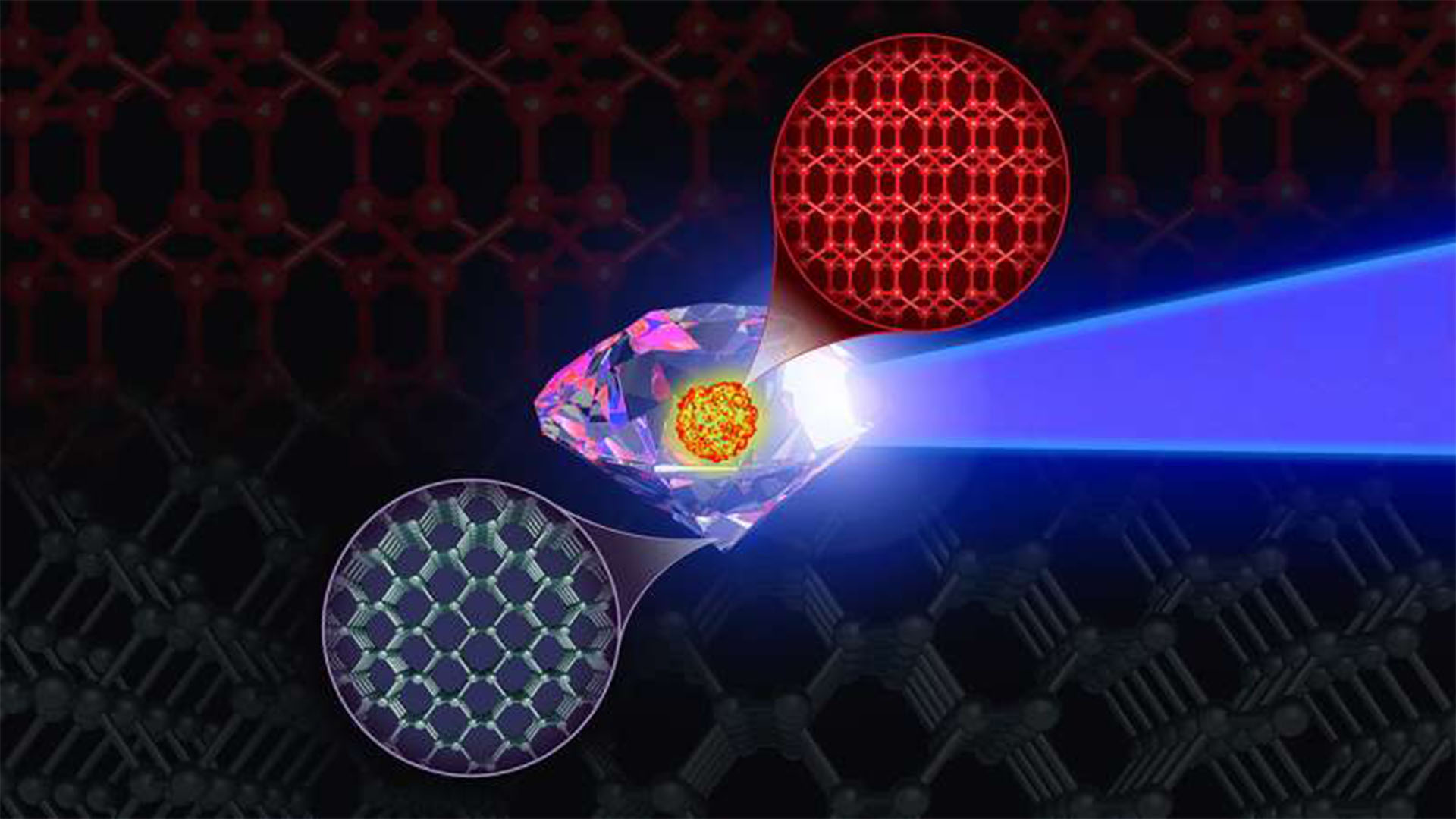
Scientists have simulated an elusive, superstrong form of carbon that may be tougher than diamonds, the hardest known material. But observing the real thing might require a trip far outside our solar system, to the center of an exoplanet — a feat that's not likely anytime soon, or possibly ever.
BC8, as the superstrong carbon is known, is an eight-atom crystal that would be 30% more resistant to compression than diamonds, according to a new study. Scientists have been trying to synthesize this crystal in the lab, without success. The new simulation reveals that the material can be made only in a narrow range of pressures and temperatures, which might make that synthesis possible in the future, researchers reported in the study, which was published in The Journal of Physical Chemistry Letters in January.
The research also helps to reveal what might be at the hearts of carbon-rich exoplanets, which are predicted to have just the right conditions for the formation of BC8.
Related: 'Mind-boggling' alloy is Earth's toughest material, even at extreme temperatures
"[T]he extreme conditions prevailing within these carbon-rich exoplanets may give rise to structural forms of carbon such as diamond and BC8," study senior author Ivan Oleynik, a physics professor at the University of South Florida, said in a statement. "Therefore, an in-depth understanding of the properties of the BC8 carbon phase becomes critical for the development of accurate interior models of these exoplanets."
In the new research, Oleynik and his colleagues used Frontier, a supercomputer at the Oak Ridge Leadership Computing Facility in Tennessee. They ran simulations of billions of carbon atoms under different pressures and temperatures to understand how these amply available atoms can transform into a material so rare, it's never been observed.
They found that BC8 is likely very stable at very high pressures of 1,250 gigapascals and above. That's well over 12 million times the pressure of the atmosphere on Earth's surface. Theory also suggests, however, that the crystal, once formed, would remain stable at ambient temperatures. BC8's atomic structure is similar to a diamond's, but it lacks diamonds' cleavage planes, the gemstones' weakest points, study co-author Jon Eggert, a scientist at Lawrence Livermore National Laboratory (LLNL), said in a statement.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Armed with their new knowledge of BC8's formation pathways and stability, the researchers are making new attempts to synthesize the material at LLNL's National Ignition Facility. These types of methods involve shocking diamonds twice at upward of 45,000 mph (72,000 km/h) and then compressing them under enormous pressures.

Stephanie Pappas is a contributing writer for Live Science, covering topics ranging from geoscience to archaeology to the human brain and behavior. She was previously a senior writer for Live Science but is now a freelancer based in Denver, Colorado, and regularly contributes to Scientific American and The Monitor, the monthly magazine of the American Psychological Association. Stephanie received a bachelor's degree in psychology from the University of South Carolina and a graduate certificate in science communication from the University of California, Santa Cruz.
 Live Science Plus
Live Science Plus