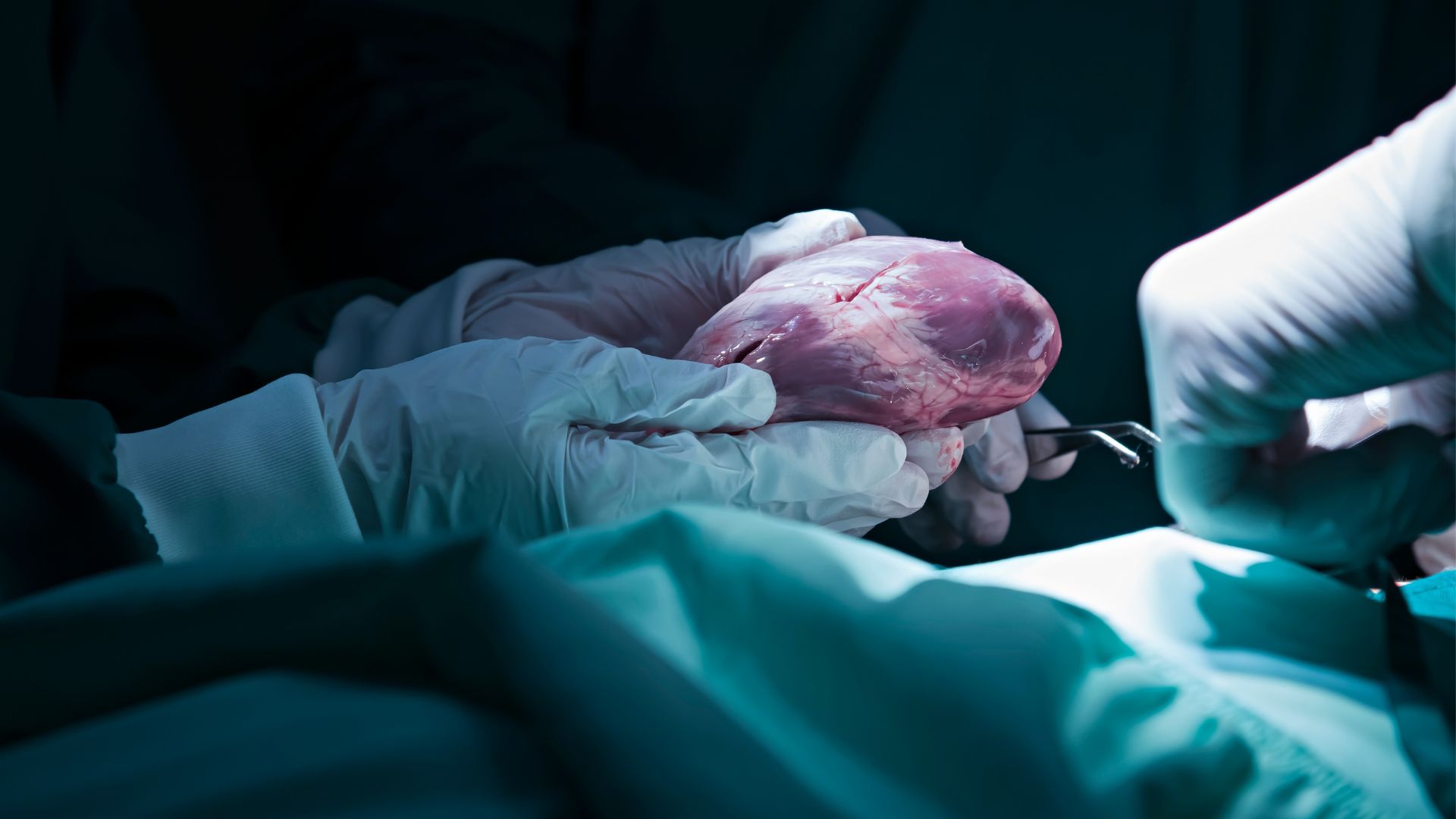
'Reanimated' hearts can be successfully transplanted and could expand donor pool
A gold-standard clinical trial suggests that "reanimating" donated hearts is a viable strategy for expanding the pool of potential heart donors.
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
A method for "reanimating" organ donors' hearts works just as well as the standard approach to collecting hearts for transplantation, new trial data shows. If widely applied, the method could increase the heart donor pool by an estimated 30%.
"Honestly if we could snap our fingers and just get people to use this, I think it probably would go up even more than that," Dr. Jacob Schroder, a transplant surgeon at the Duke University School of Medicine who led the trial, told The Associated Press. "This really should be standard of care."
Most transplanted hearts come from brain-dead donors, who've died due to a complete loss of brain activity, rather than circulatory death, in which the heart stops. A brain-dead patient can be declared dead before their heart stops beating, and this allows doctors to remove the heart while it's still perfused with oxygen-rich blood and therefore not yet damaged by a lack of oxygen. The heart is then flushed with a preservation solution, placed in an ice-filled cooler and rushed to its recipient.
In the recent trial results, published Thursday (June 8) in The New England Journal of Medicine, doctors compared the survival rates of transplant recipients who got hearts from brain-dead donors with the survival rates of those who got hearts from donors who'd died of circulatory death. A total of 166 U.S.-based heart transplant patients were included in the primary analysis — 86 in the brain-death group and 80 in the circulatory-death group.
Related: The 9 most interesting transplants
Six months post-surgery, the brain-death group had a 90% survival rate and the circulatory-death group had a 94% survival rate, suggesting that donation after circulatory death, or DCD, is an equally viable approach to heart transplants.
DCD is made possible by "extracorporeal machine perfusion," which involves hooking up the donor organ to a machine that pumps blood and nutrients through its tissues. The new trial tested a perfusion system called Organ Care System Heart, made by TransMedics, the trial's funder. The system warms the blood that it pumps through the donor hearts, as compared with other perfusion systems, which still require the organ be cooled as part of the preservation process.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Although the two groups had similar survival rates at six months, compared with the traditional heart transplant group, the DCD group had slightly higher rates of moderate to severe primary graft dysfunction, in which one or both of the heart's ventricles show dangerous dysfunction within 24 hours of the transplantation surgery. However, none of the DCD patients had primary graft failure that resulted in retransplantation, while two people in the traditional transplant group did.
Overall, the rate of serious adverse events was very low and similar in both groups, as assessed out to 30 days post-surgery.
The new trial involved multiple medical centers and builds upon previous evidence in favor of DCD, which mostly drew from isolated cases and small trials performed at single centers in Australia and the United Kingdom, the trial authors wrote.
Historically, though, one big hurdle to using perfusion systems on hearts and other donated organs is cost. Back in 2019, Dr. Brian Lima, director of cardiac transplantation at the Sandra Atlas Bass Heart Hospital in Manhasset, New York, told Live Science that few studies had compared perfusion with standard cold storage, so no "earth-shattering data" existed that could convince hospitals to make the expensive switch nationwide.
However, at that time, doctors at Duke University Medical Center had just performed the nation's first adult heart transplant with a "reanimated" organ. Following that landmark procedure, Lima suggested that perfusion could soon become the standard of care.

Nicoletta Lanese is the health channel editor at Live Science and was previously a news editor and staff writer at the site. She holds a graduate certificate in science communication from UC Santa Cruz and degrees in neuroscience and dance from the University of Florida. Her work has appeared in The Scientist, Science News, the Mercury News, Mongabay and Stanford Medicine Magazine, among other outlets. Based in NYC, she also remains heavily involved in dance and performs in local choreographers' work.
 Live Science Plus
Live Science Plus