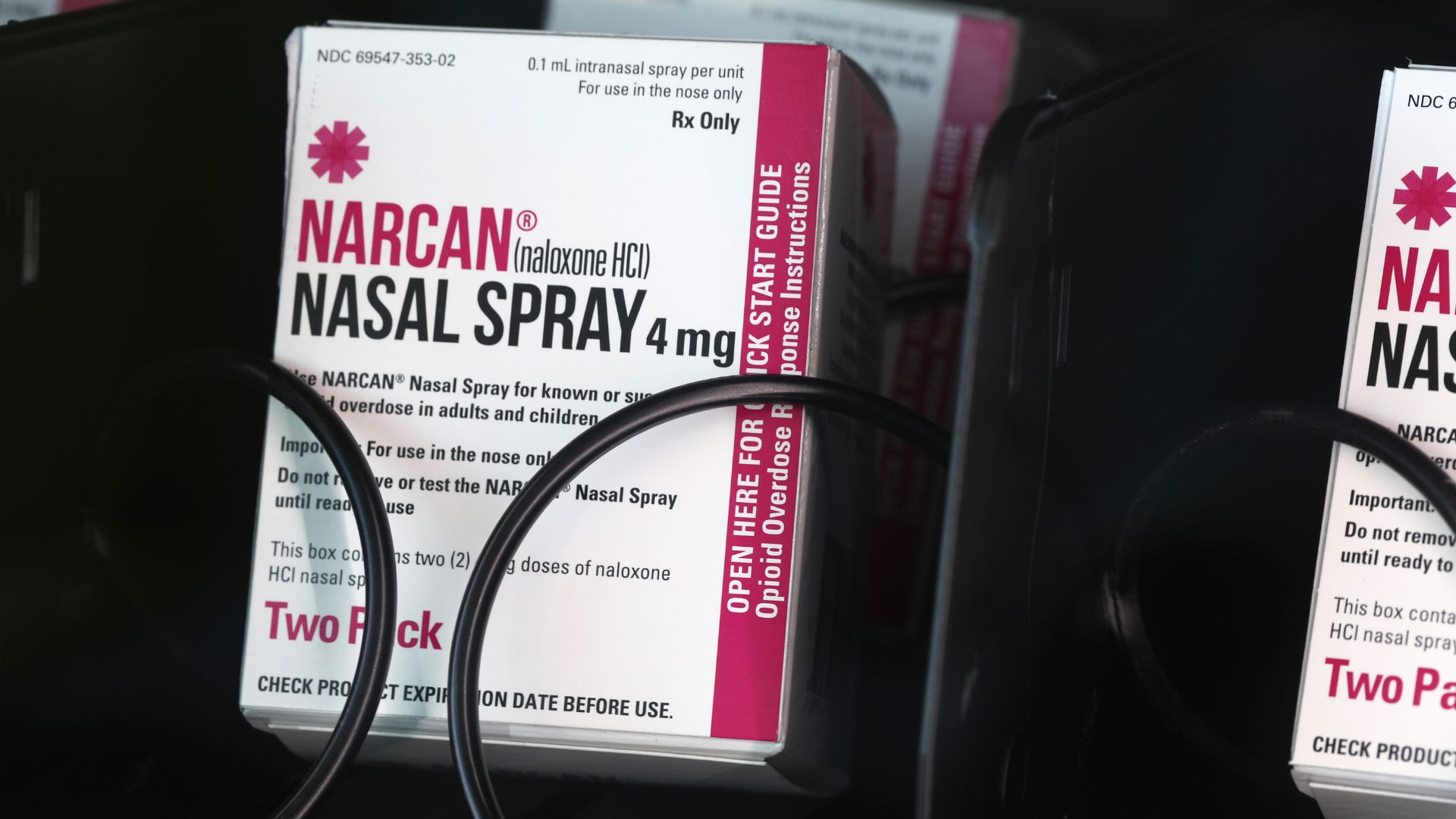
FDA approves over-the-counter Narcan to combat opioid overdose crisis
The U.S. Food and Drug Administration has approved a naloxone nasal spray for over-the-counter use.
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
For the first time, the U.S. Food and Drug Administration (FDA) has approved a naloxone product — a medication that rapidly reverses the effects of opioid overdose — that can be purchased over-the-counter.
The medication, Narcan, is a nasal spray that was approved as a prescription drug back in 2015, according to the FDA. To change the drug's status, the manufacturer provided data demonstrating that Narcan is safe and effective when used as directed and can be safely used by consumers without supervision. Based on this data, an advisory committee unanimously recommended that the drug be cleared for over-the-counter use, and the FDA has now acted on this recommendation.
All 50 states have "Narcan access laws" on the books that already allow pharmacists to hold a standing prescription for the drug and thus dispense Narcan or a generic equivalent to anyone who requests it — but many pharmacies chose not to do so, according to The New York Times. Many community-based organizations and local health departments do offer the drug at little to no cost, according to the Centers for Disease Control and Prevention (CDC).
Related: Opioids are the most common cause of fatal poisonings in kids under 5
The approval of over-the-counter Narcan is expected to greatly improve access to the life-saving drug, which can revive a person experiencing an opioid overdose within two to three minutes.
"Today's approval of OTC naloxone nasal spray will help improve access to naloxone, increase the number of locations where it’s available and help reduce opioid overdose deaths throughout the country," Dr. Robert M. Califf, the FDA Commissioner, said in the agency's statement. "We encourage the manufacturer to make accessibility to the product a priority by making it available as soon as possible and at an affordable price."
The CDC offers simple instructional videos on how to use naloxone nasal spray, as well as how to use injectable naloxone, another version of the medication.
Get the world’s most fascinating discoveries delivered straight to your inbox.

Nicoletta Lanese is the health channel editor at Live Science and was previously a news editor and staff writer at the site. She holds a graduate certificate in science communication from UC Santa Cruz and degrees in neuroscience and dance from the University of Florida. Her work has appeared in The Scientist, Science News, the Mercury News, Mongabay and Stanford Medicine Magazine, among other outlets. Based in NYC, she also remains heavily involved in dance and performs in local choreographers' work.
 Live Science Plus
Live Science Plus





