Simulating the Heart to Improve Cardiac Treatments

Editor's Note: This article has been updated to correct and clarify research characterizations within the original text.
This Behind the Scenes article was provided to LiveScience in partnership with the National Science Foundation.
In the United States, almost half a million people die every year, because their heart beats too fast or beats too slow — a disease called cardiac arrhythmia. Although researchers and doctors have taken great strides to understand the heart, cardiovascular disease is still the primary cause of death in the industrialized world.
Scientists have long developed cardiac therapies through experience-based experimentations, which is often through trial and error. Yet, a new way of studying the heart can perhaps provide new avenues to developing improved cardiac treatments. Together with other researchers, Ellen Kuhl, professor at Stanford University, studies the heart through a simulation-based predictive method.
"By simulating the heart, we can better understand the complex pathways of cardiac disease. This can help us to improve current treatment strategies," Kuhl said.
Simulating the heart
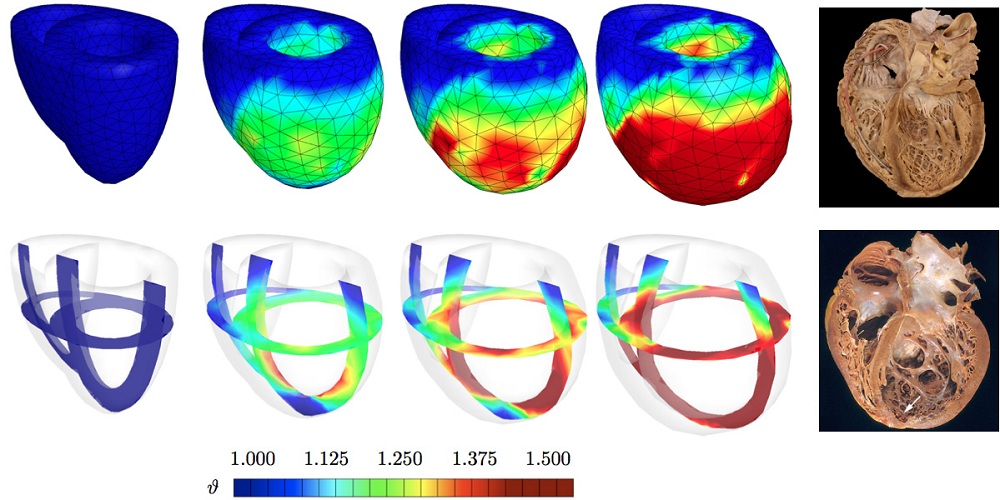
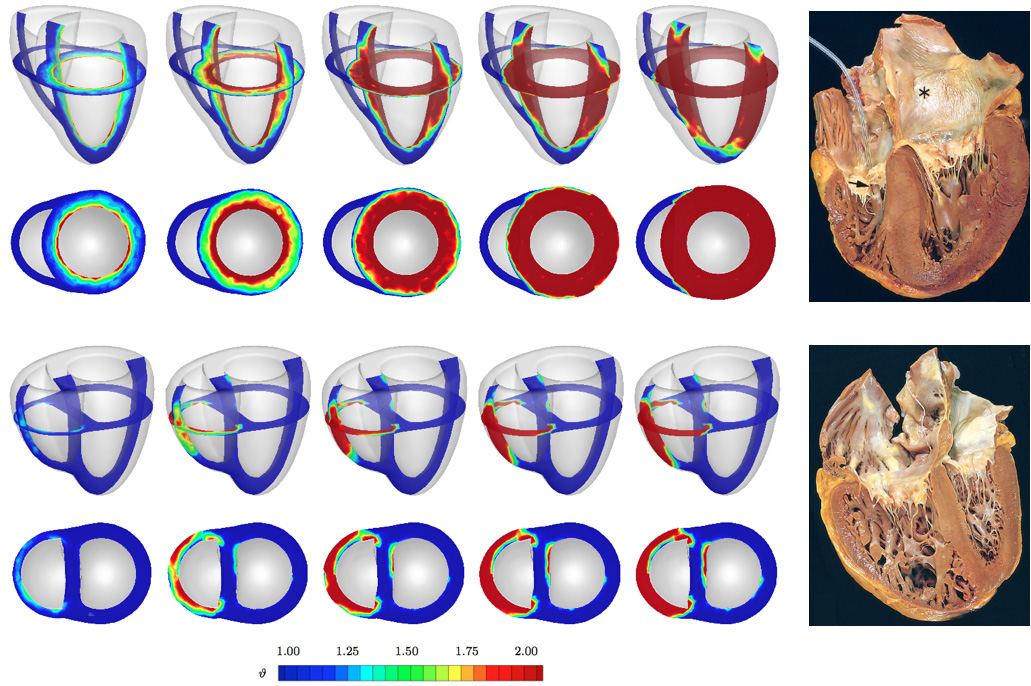
Kuhl and her team built a computational model of a student's heart, simulating how a real heart works — a phenomenon where the flow of sodium and potassium controls the heart's electrical charge, which in turn, causes the heart to contract and pump blood all over the body.
Get the world’s most fascinating discoveries delivered straight to your inbox.
This simulation-based predictive method combines the implementation of new advanced continuum theories, modern imaging modalities and computational techniques. The idea is that if we can simulate a heart, we can predict it, better understand it, and thus treat it more effectively.
"It would be a huge step forward if we could provide a true mechanistic understanding on how different interventions alter the interplay of physical fields that characterizes the dynamics of the heart," said Kuhl. "It would allow us to virtually probe all kinds of different treatment scenarios just by a mouse click."
Heart light
In order to simulate a live human's heart by creating a computational model of the heart, the team used equations to establish a computational algorithm that can reliably predict the excitation-contraction patterns of a healthy heart. The electrocardiogram, a test that records heart electrical activity, of the real heart agrees nicely with that predicted by the computational model.
"This is exciting as it allows us to predict what would happen if we, for example, manipulate signal propagation or pace the heart externally," said Kuhl. The vision is to treat the patient with stem cell therapies based on injecting cells into the damaged heart tissue to restore function.
Already, the computational method has proposed treatment improvements for some cardiac diseases. When a patient suffers from cardiac arrhythmia, the current gold standard is the use of pacemakers. Pacemakers must sit on the heart in order to pace the heart with electrical signals. Unfortunately, they tend to fail overtime due to the wear and tear of the hearts movement. Kuhl's success in simulating the heart together with first prototype experiments by her collaborator Oscar Abilez, have led to an innovative way to pace the heart: Using light.
Not only would doctors be able to predict different outcomes upfront rather than having the patient come many times a month to find the optimal pacing sequence, but Kuhl said that "this would allow us to pace the heart with very high precision from a distance, unlike now, where pacing is done with electrical pace makers that have to sit on a constantly moving heart muscle."
Stalling heart failure
This methodology can also improve yet another form of cardiac disease. Today, treatment for patients suffering from myocardial infarction, interruption of blood supply to the heart, is limited. The latter is caused by the local death of heart muscle cells making the heart incapable of contracting. In the future, patients might benefit from stem cell therapy, which aims to repair damaged tissue.
Towards that goal, Kuhl and her team have been able to simulate the progression of infarct-induced heart failure, allowing them to predict optimal cell injection sites. Such prediction would make stem cell therapy more reliable and effective.
"The methods we use — predictive, quantitative, computational models — might change the way we design, improve and optimize medical treatment," said Kuhl. "There is a long way to go, and it is exciting to be a part of these developments."
Educational hearts
These developments have also been exciting for Kuhl's team, which includes several undergraduate students from Stanford University. Corey Murphey, for example, has been involved in asking critical questions and providing important feedback for model adjustment and improvement.
"My work with Ellen has helped me realize what I really like doing and has given me some fantastic opportunities (e.g., being able to go to national and international conferences)," said Murphey.
Heart simulations present great insights to understanding the heart. In the future, with the advancement of computational techniques and other technologies, we might have a set of disease specific algorithms that could be translated into effective forms of treatment. Perhaps a different approach of study is just what we needed.
Editor's Note: This research was supported by the National Science Foundation (NSF), the federal agency charged with funding basic research and education across all fields of science and engineering. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author and do not necessarily reflect the views of the National Science Foundation. See the Behind the Scenes Archive.