Physicists 'See' Location of 23,000 Single Atoms for First Time
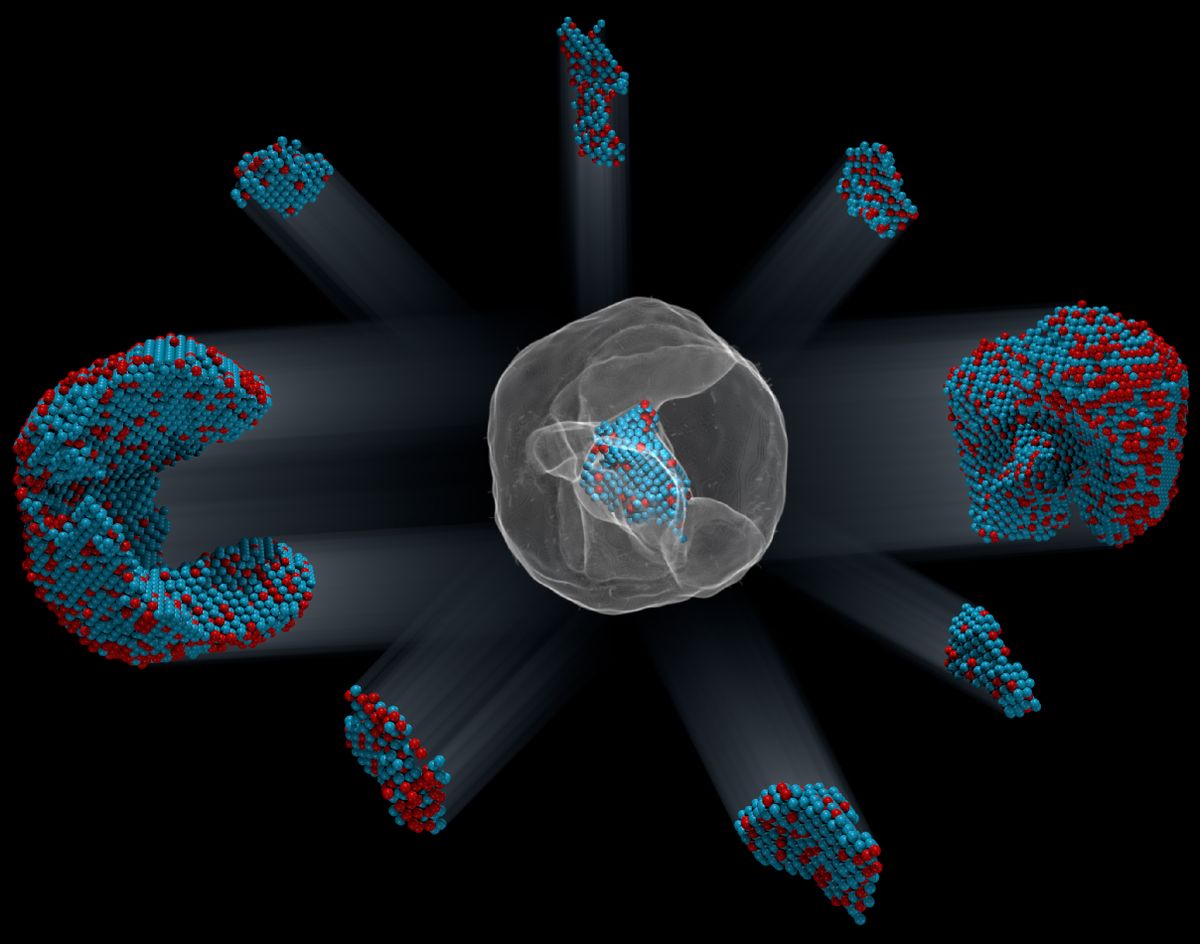
For the first time, scientists have seen the exact locations of more than 23,000 atoms in a particle that's small enough to fit inside the wall of a single cell.
A team led by Peter Ercius of Lawrence Berkeley National Laboratory and Jianwei Miao of UCLA used a scanning electron microscope to examine a particle that was made of iron (Fe) and platinum (Pt) that was only 8.4 nanometers across, they reported yesterday (Feb. 1) in the journal Nature. (A nanometer is a billionth of a meter, or 3.9 one-hundred-millionths of an inch.)
Why would anyone care about the location of each little atom? "At the nanoscale, every atom counts," Michael Farle, a physicist at the University of Duisburg-Essen in Germany, wrote in an accompanying News and Views article in Nature. "For example, changing the relative positions of a few Fe and Pt atoms in a FePt nanoparticle dramatically alters the particle's properties, such as its response to a magnetic field." [Images: Tiny Life Revealed in Stunning Microscope Photos]
Electron beams
Using a scanning electron microscope, a beam of electrons is passed over the surface of an object to create an image. That allows researchers to see even small details of tiny bits of material like crystals and protein molecules. "There are very powerful techniques for figuring out the structure of crystals," he said. "But those have to be perfect crystals."
Ordinarily, when this kind of electron microscope is used to look at a crystal or other large molecule, the electrons are beamed at the sample and they scatter as they hit it, rather like a stream of bullets fired from a machine gun would scatter off Superman's chest. After they bounce off the atoms, the electrons hit a detector, and from there, the researcher can look at where the electrons land to get a look at the arrangement of the atoms in the crystal or molecule.
The problem, Ercius said, is that the image is built from an average that's obtained using many atoms or molecules. That is, the researchers will see a pattern, but it can only tell that person what the bulk arrangement of the atoms is, not where each one is actually located. [Image Gallery: Stunning Peek Inside Molecules]
The iron-platinum nanoparticles are a kind of irregular crystal. But the ordinary scanning method wouldn't work as well for them, because the atoms are arranged in unique and slightly irregular ways, the researchers said. So they had to find a new way to use the electron microscope: They decided to look at the sample iron-platinum particle from many different sides.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Locating single atoms
To do that, they altered the way the sample was prepared. Instead of leaving it in place, they put it on a special base that let them rotate and tilt their particle of iron and platinum, changing its orientation slightly after each "snapshot" with the electron beam. Otherwise, the process the researchers used was the same as usual.
That simple change was powerful: The varying orientations produced different patterns of scattering. The different patterns, which were picked up on a detector that's similar to the ones in digital cameras, could be used to calculate the exact positions of the 6,569 iron and 16,627 platinum atoms in the nanoparticle. It's not unlike making a 3D model of an object by taking pictures from many angles, which animators do routinely. Their results for the atoms' locations reached a resolution of about one-tenth the diameter of a single atom, according to Farle.
In the future, getting such an accurate picture could aid materials scientists in creating nanometer-size structures for applications such as hard drives. Makers of hard drives want to fabricate tiny, near-perfect crystals so that they can be easily magnetized and will hold a magnetic field for a long time, Ercius noted.
"All crystals have defects," Ercius said. "The problem is when they get nanoparticles that have these weird defects in them. This means they can look at those and how they affect how things work."
Knowing each atom's exact location would also allow scientists to predict how a crystal might grow. Ercius noted that right now, when materials scientists run simulations, they have to assume that a crystal grows in a certain way, and those assumptions guide their predictions for the future. If they could see exactly where the atoms are, they could make more accurate predictions of what the crystal will look like when it has grown to full size.
"What's so good about this is it measures disorder," Ercius said. "It lets you see unique objects."
Original article on Live Science.

 Live Science Plus
Live Science Plus