New Zika Vaccine: Testing in People Underway in US
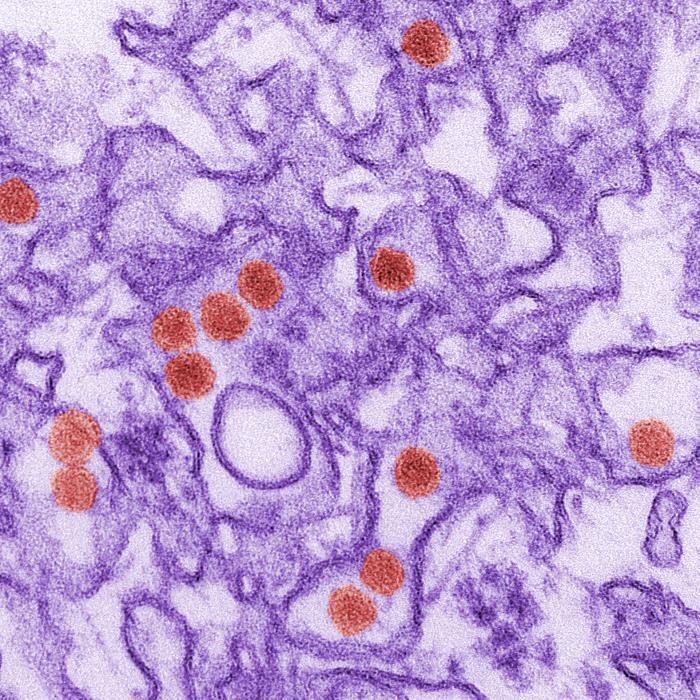
A new vaccine against the Zika virus is being tested in people, and researchers said they hope to have early results by the end of the year, officials said today.
Scientists have just started giving the vaccine to the first few volunteers as part of an early study run by the National Institute of Allergy and Infectious Diseases (NIAID). In total, 80 people in the U.S. ages 18 to 35 will receive the experimental vaccine during the trial.
The study is intended to determine the safety of the vaccine, called the NIAID Zika virus investigational DNA vaccine, as well whether it holds potential to protect people from Zika infections. If the vaccine appears safe and produces a good immune response against the virus, researchers plan to start a larger study, known as a Phase 2 trial, in early 2017 in countries where Zika outbreaks are occurring, said Dr. Anthony Fauci, director of NIAID.
"NIAID has worked rapidly to accelerate the process" and move the vaccine from animal testing into a clinical trial, Fauci said in a news conference today (Aug. 3). "If we get a good immune response [from the vaccine] and there are no safety red flags … we should know if it's OK to move on to Phase 2," Fauci said. [Zika Virus News: Complete Coverage of the Outbreak]
The vaccine contains a small, circular piece of DNA called a plasmid. Within that plasmid are genes that code for certain Zika virus proteins. These, in theory will cause people who are injected with the vaccine to mount an immune response against the virus, thus protecting them from infection, the researchers said. This type of vaccine is known as a DNA vaccine, and researchers have used it in the past for diseases such as West Nile virus. The vaccine doesn't contain infectious proteins, so people will not get an actual Zika infection from the vaccine, the researchers said.
Everyone in the new study will get at least one dose of the new Zika vaccine, and some participants will get two or three doses that are spaced out over a number of weeks. The vaccine is given with a needle-free "jet injector," which uses a high-pressure stream of fluid to inject the vaccine into the muscle.
Although researchers expect to collect data on the vaccine's safety and effectiveness soon after the trial begins, they will continue to follow the participants for two years to measure how long their immune response lasts, Fauci said.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Fauci noted that it could take several years before this vaccine would be ready to use outside of a clinical trial.
If the vaccine eventually becomes approved, it's not yet clear exactly who would get it, or when. The target population for the vaccine is women of childbearing age, Fauci said, because the Zika virus can cause birth defects if women get infected during pregnancy.
But the vaccine would not be given during pregnancy, in part because the safety of receiving the vaccine during pregnancy is not being tested, and because pregnant women can get Zika even before they know they are pregnant. "You want to protect someone before they get pregnant," Fauci said.
It's possible that, in Zika-affected countries, doctors will end up giving an approved Zika vaccine to all children, as was done with the vaccine against rubella, a disease that is also linked to birth defects in pregnancy, Fauci said. (Many vaccines are given in childhood to protect children against diseases at the earliest possible age.)
In addition to the new vaccine that this trial is investigating, several other vaccines against Zika are also in the works. Last month, Inovio Pharmaceuticals announced that it had started a clinical trial with another DNA vaccine against Zika. The drug company GlaxoSmithKline is also preparing to test a new Zika vaccine that uses a different technology, called a self-amplifying mRNA vaccine. And researchers at the Walter Reed Army Institute of Research are working on a Zika vaccine that uses a killed strain of the virus.
Original article on Live Science.

Rachael is a Live Science contributor, and was a former channel editor and senior writer for Live Science between 2010 and 2022. She has a master's degree in journalism from New York University's Science, Health and Environmental Reporting Program. She also holds a B.S. in molecular biology and an M.S. in biology from the University of California, San Diego. Her work has appeared in Scienceline, The Washington Post and Scientific American.