Lab-Grown Esophagus Could Aid Cancer Patients
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
Doctors have implanted bio-engineered tracheas in patients, and researchers have experimented with growing bladders and kidneys. Now, another organ joins that list: the esophagus, which brings food and water to the stomach.
An international team of scientists working at Kuban State Medical University in Krasnodar, Russia, has built a working esophagus from stem cells, and implanted the organ into rats, the researchers say. The new esophagus functioned just as well as the rats' natural organs, said the researchers, who detailed their work today (April 15) in the journal Nature Communications.
Every year, about 18,000 people in the United States are diagnosed with esophageal cancer, and others suffer from congenital defects, or are injured after medical procedures or swallowing caustic materials. Many of these cases require surgery, which can involve taking a section of the small intestine or the stomach to replace part of the esophagus.
Unfortunately, this isn't always the best solution. Patients can suffer complications, and many still have trouble swallowing solid food after surgery. [5 Crazy Technologies That Are Revolutionizing Biotech]
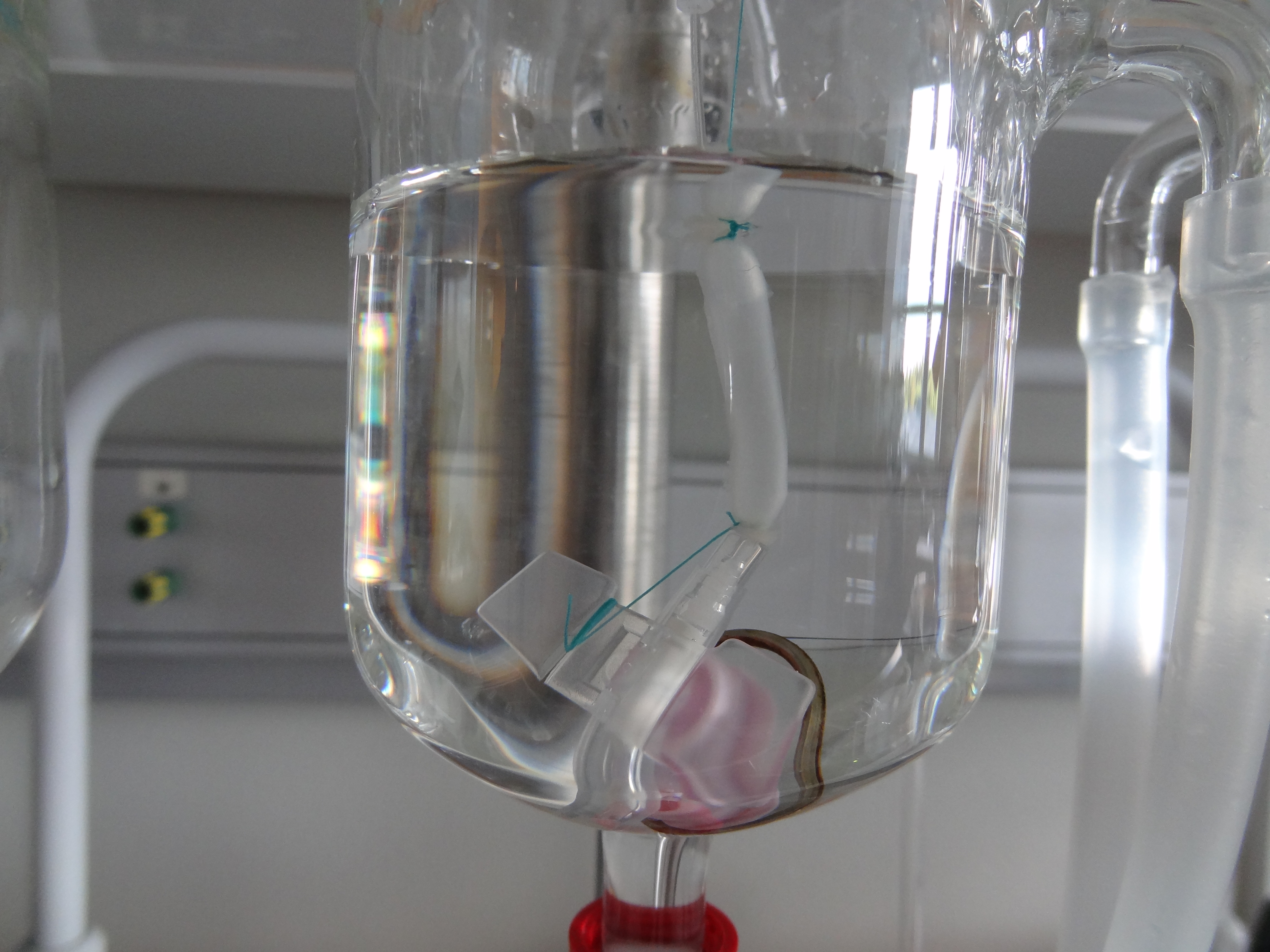
Researchers led by Paolo Macchiarini of the Karolinska Institutet in Stockholm took a section of a rat's esophagus and removed the cells, leaving behind a scaffold of protein. Such "decellularization" is now a common technique for making structures for cells to latch onto when doing regenerative organ experiments.
To test whether the scaffold would be strong enough to stand up to repeated cycles of expansion and contraction, the scientists pumped air into it 10,000 times, allowing it to blow up and shrink.
The researchers then took stem cells called allogeneic mesenchymal stromal cells, which don't cause an immune reaction when implanted into tissue. Scientists placed these cells on the scaffold, allowing the esophagus to grow for three weeks.
Get the world’s most fascinating discoveries delivered straight to your inbox.
They then implanted the esophagus into a rat, replacing up to 20 percent of its esophagus with the engineered version. They repeated this procedure in nine more rats.
Researchers kept the rats on a liquid diet for a week, and then gave them soft foods the week after that. The rats didn't suffer any problems and survived longer than those that underwent a sham surgery, as a control.
Macchiarini said the scaffold provides structure for the stem cells, as well as the chemical cues that tell them what kind of cells they should develop into.
When an animal swallows, the involuntary and voluntary nerve impulses have to work together in the correct way. That means that in order to work properly, an esophagus has to develop muscle cells, connections to the nervous system and blood vessels. "We were really surprised at the level of differentiation we got," Macchiarini told Live Science.
Although this technique for building an esophagus seems to work in rats, there is still a long way to go before it could be tested in people. Differences between rats and humans could complicate the translation. For example, rat and human esophagi don't have exactly the same types of muscles and structure.
There's also the question of whether growing an esophagus could be scaled up, as a rat esophagus is much smaller than a person's.
In the next steps, Macchiarini said, researchers will need to move to experiments in larger animals, as well as with other organs, to see if the concept they tried in this study is broadly applicable.
Follow Live Science @livescience, Facebook & Google+. Original article on Live Science.

 Live Science Plus
Live Science Plus










