Green Gasoline Comes Closer to Fueling Your Car

This Behind the Scenes article was provided to LiveScience in partnership with the National Science Foundation.
The backbone of our energy infrastructure is carbon-based fuel. In the form of oil, coal and natural gas, carbon runs our cars, heats our homes and cooks our food. We can minimize the shock of transitioning away from fossil fuels to sustainable sources by using as much existing carbon-based infrastructure as possible.
Plants are the only source of sustainable carbon, in that there are no other means to simply capture carbon from the air to make carbon-based fuel. That is the beauty of plant-based biofuels and why they must have a role in our sustainable-energy future.
All life is carbon based. To convert plant-based carbon into fuels we can use in our cars, we must first understand the composition of the plants.
Plants as energy
First, think of how your body stores energy as fat but is structurally composed of a different type of molecule called protein; plants have a similar dichotomy. A plant stores energy as starch, sugar and fat, but is structurally composed of lignin and cellulose (or 'lignocellulose'). Take corn as an example: from kernels comes starch and sugar that can be fermented to make ethanol; corn oil can be used to make biodiesel.
While those technologies deserve study in their own right, we mustn't ignore the remaining cobs, husks and stalks — the structural materials made of lignin and cellulose. Wood and grass are made of the same structural materials.
Get the world’s most fascinating discoveries delivered straight to your inbox.
The structural materials of plants are the cheapest and most abundant forms of biomass available — plus humans can't eat them (cows and beavers can, only because their guts have special bacteria). Fuel made from inedible material has the benefit of never directly interfering with food production.
Green gasoline
Research conducted at the University of Massachusetts, Amherst headed by George Huber focuses on converting sawdust and switchgrass into "green gasoline" — a fuel that we can use within our current infrastructure. In a few years you may start seeing it blended with what you buy at the pump.
"Unless there's a sign that tells you so, you won't even know your gas is 'green,'" said Huber. "It has zero carbon footprint. The CO2 [carbon dioxide] from your car is recycled to the plant when it re-grows."
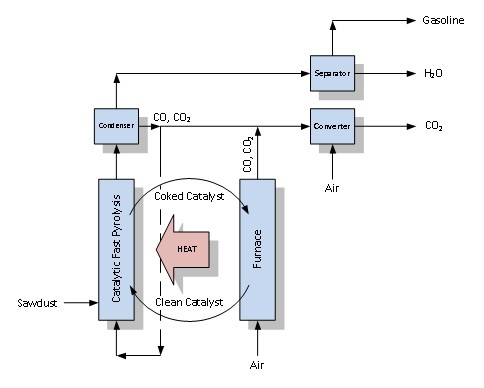
Green gasoline is made through a technique called catalytic fast pyrolysis in a fluidized bed. Let's break this process down by the terms and techniques used:
'Catalytic'
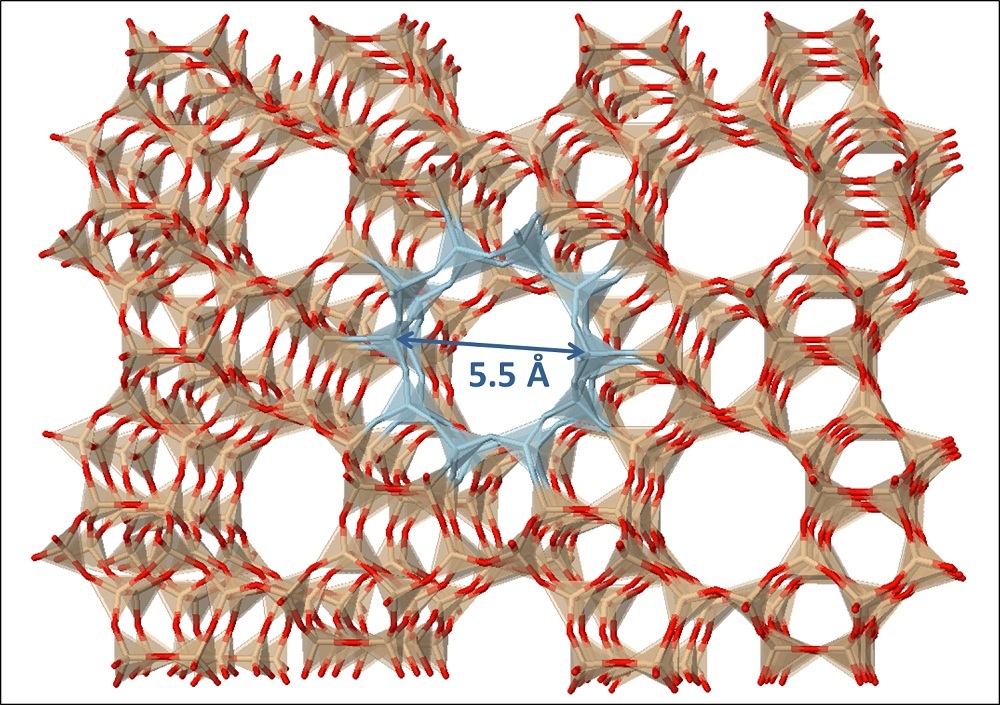
A catalyst is a material used to make a specific chemical reaction happen quicker than it normally would. The reaction in question? Turning lignocellulose into gasoline. The catalyst that does that is a special class of materials called "zeolites," materials made of silicon, aluminum and oxygen atoms that are arranged in intricate geometrical frameworks in a crystal-like pattern.
Spaces between the molecules held together in these frameworks are called micropores and hold carbon-based molecules on the catalyst surface in an arrangement that causes them to form molecular products of the same size. The trick is to select a zeolite with a pore size equal to that of gasoline molecules. The catalysts the Huber lab uses are cheap materials currently used in the in the petrochemical industry.
'Fast pyrolysis'
Pyrolysis is the high-temperature breakdown of large molecules (such as cellulose or plastic) into small-molecule fumes. The pyrolysis is "fast" because quick heating is necessary to turn the plant carbon into fumes instead of char. "Slow pyrolysis" is how charcoal is made.
In a camp fire, the flames you see are actually wood-pyrolysis fumes reacting with oxygen. Flame heat causes the log to pyrolize more and maintains itself until the log's surface becomes too covered in char to produce more fumes. In the lab, pyrolysis of sawdust is done in the absence of oxygen so the fumes can't burn like they do in a fire — the fumes instead react on the catalyst surface to form gasoline.
'Fluidized Bed'
Fluidization is a technique for maximizing contact and mixing between the gas fumes from the pyrolysis and solid catalysts. Envision how a bed of gravel dances beneath the end of a syphon tube when cleaning a fish tank; the gravel is fluidized by the water. In the lab, the role of gravel is assumed by a bed of sand-like catalyst particles and sawdust undergoing pyrolysis. The water is replaced with a stream of hot gas entering from the bottom, called a "fluidizer." Vapors leaving the catalyst surface get blown out of the reactor where they are condensed as fuel with an octane rating of 108.
A catalytic fast pyrolysis chemical plant
Because lignocelluose contains more oxygen than gasoline, a portion of the carbon (roughly 25 percent) must leave as carbon monoxide and carbon dioxide.
At scales larger than in the lab, a fraction of the stream will be used as the fluidizer. The remaining 75 percent is theoretically available for producing gasoline, but char cuts into this number and forms a black coat on the catalyst particles.
For the reaction to run for long periods, particles of charred catalyst must be recycled through a furnace where char is burned off. The furnace creates enough heat to power catalytic fast pyrolysis. In that way, 30 percent of the carbon from the sawdust fed into the system leaves the facility as gasoline.
It takes 45 pounds of sawdust to make a gallon of gasoline. However, notice how a facility using the technology needs no inputs other than the sawdust and air — the technique would be ideal for isolated, off-the-grid locations where lots of plant materials are available.
Onward
Pilot-scale tests have proven successful, and will be scaling up into a demonstration chemical plant soon.
"This is a great time to be in this field. As the price of oil increases it gives opportunities to future engineers," said Huber. "It's up to the new generation to find solutions for the future."
For more information, visit the Huber research-group Web site.
Editor's Note: This research was supported by the National Science Foundation (NSF), the federal agency charged with funding basic research and education across all fields of science and engineering. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author and do not necessarily reflect the views of the National Science Foundation. See the Behind the Scenes Archive.
Robert Coolman, PhD, is a teacher and a freelance science writer and is based in Madison, Wisconsin. He has written for Vice, Discover, Nautilus, Live Science and The Daily Beast. Robert spent his doctorate turning sawdust into gasoline-range fuels and chemicals for materials, medicine, electronics and agriculture. He is made of chemicals.