Inside a Molecule: New Image Reveals Surprising Physics
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
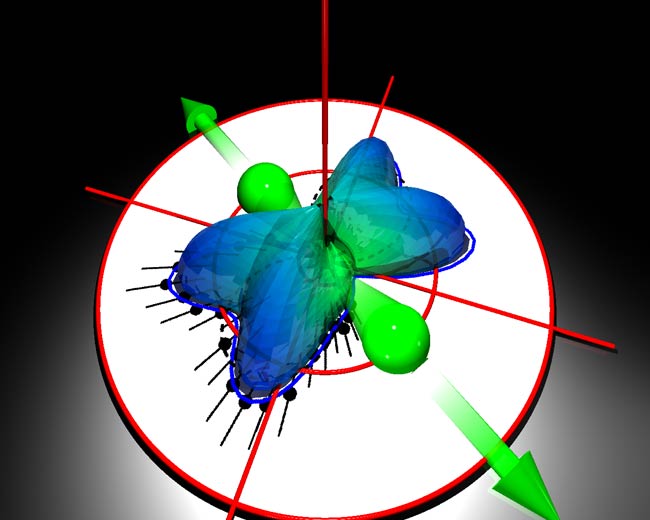
To get a good picture of a molecule, you have to get it to explode. By doing so, an international team of researchers has made the clearest snapshot yet of one of Nature's tinier entities.
The new picture reveals some surprising small-scale physics.
"You cannot feel inside a molecule," explained Thorsten Weber from Lawrence Berkeley National Laboratory (LBNL). Instead, "you have to let it explode and then track back where things came from."
Weber and his colleagues used the Advanced Light Source at LBNL as a camera flash. This high-powered, pulsed laser beam strips negatively charged electrons from molecules. Without the electrons, the molecule's positively charged nuclei tend to fly apart.
Weber compared the laser beam to a sharp knife that snips the bonds of a molecule, so that it can unravel cleanly without losing too much of the original information. There are other ways to probe molecules that are more like hitting them with a hammer.
Motion microscope
The researchers cut open deuterium molecules. Deuterium is a heavy form of hydrogen, with a nucleus of one neutron and one proton. Two of these nuclei - separated by a short distance and surrounded by two electrons - make up a molecule.
Get the world’s most fascinating discoveries delivered straight to your inbox.
The electrons and nuclei can be pulled apart from each other by the action of the laser. Inside the experiment's momentum spectrometer, a configuration of electric and magnetic fields guides the freed particles to detectors that act as the film of the camera.
"The challenge is that you have to measure four particles at the same time," Weber told LiveScience. His team's spectrometer is an improvement upon previous set-ups in that it captures particles no matter what direction they fly out.
By reconstructing the trajectories of the molecular shrapnel, the scientists were able to determine what the molecule looked like, specifically how things were moving inside it, before the laser hit.
"We know that nothing in the world stands still," Weber said. "Our spectrometer is a microscope of motion."
Surprise inside
Seeing the internal dynamics of the molecule with such detail revealed a surprise. As described in a recent issue of Nature, Weber's team found that the movement of the two electrons depended on the distance between the nuclei in a way not predicted by theory.
Weber explained that theorists who study molecules have to make approximations when dealing with more than two particles - otherwise there is just too much to calculate. The fact that an unexpected result turned up in the relatively simple deuterium molecule may mean that some of these theoretical assumptions may come under increased scrutiny.
The chemical properties of a molecule - like the angle between two bonds, or the frequencies at which light is absorbed - often cannot be predicted directly from fundamental laws. But Weber thinks that his team's snapshots of the internal motion of molecules may uncover the underlying physics.
"We are working on the threshold of physics and chemistry," he said.
Weber and his colleagues look forward to exploring more biologically significant molecules like water and carbon dioxide with their technique. With more accurate pictures, Weber foresees a time when scientists design useful molecules for medicine and industry from the ground up.
 Live Science Plus
Live Science Plus











