How Ice Melts: Longstanding Mystery Solved
Until now, scientists could not explain why ice cubes in your drink melt. They've known the basics, but the details remained elusive.
A breakthrough new study, announced today, supports a leading theory that melting starts when the fundamental structure of matter begins to crack.
Melting is considered a basic phenomenon in physics. An understanding of how it works is crucial to gaining a firm grasp on the physical world.
"Yet major details about the mechanisms that drive the melting of an ice cube are missing," said Arjun Yodh of the University of Pennsylvania. "Superficially, the principle is straightforward. As a solid heats up, molecules within the ice acquire more energy and jiggle around more, driving the transition from a solid to a liquid. This is true in part, but reality is richer and more complex."
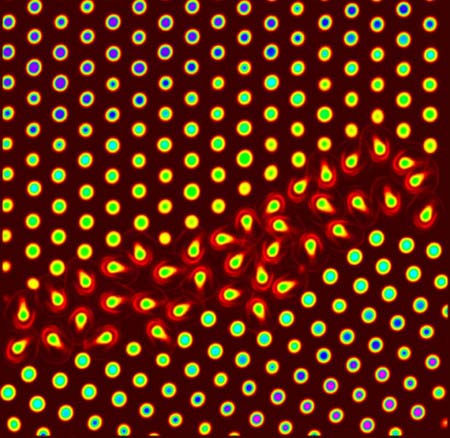
The problem is that the earliest phase of melting has never been seen. Scientists can't see the atoms involved because they are so small and because they are hidden in the structure of solid material.
So Yodh's team made some big atoms. Specifically, they made see-through crystals that are like small beads and are visible in an optical microscope.
"The spheres swell or collapse significantly with small changes in temperature, and they exhibit other useful properties that allow them to behave like enormous versions of atoms for the purpose of our experiment," said Ahmed Alsayed, a University of Pennsylvania doctoral student and lead author of a paper on the results in the July 1 issue of the journal Science.
Get the world’s most fascinating discoveries delivered straight to your inbox.
A premelting occurs in spots where atoms within solid crystals are not perfectly aligned, and they begin to move. The changes are seen in pictures taken as the material was heated. The imperfections are much like the differences seen in wood grain, the scientists said.
"These motions then spread into the more ordered parts of the crystal," Alsayed said. "We could see that the amount of premelting depended on the type of crystal defect and on the distance from the defect."
Nature could inspire technology as the process is investigated further.
"The existence of premelting inside solid materials implies that liquids exist within crystals before their melting temperature is reached," Yodh said. "Understanding this effect will provide insight for the design of strong materials that are more or less impervious to temperature changes and could also apply to our theories of how natural materials, such as water, evolve in our environment."
Robert is an independent health and science journalist and writer based in Phoenix, Arizona. He is a former editor-in-chief of Live Science with over 20 years of experience as a reporter and editor. He has worked on websites such as Space.com and Tom's Guide, and is a contributor on Medium, covering how we age and how to optimize the mind and body through time. He has a journalism degree from Humboldt State University in California.
 Live Science Plus
Live Science Plus






