Pfizer Recalls Birth Control Pills
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
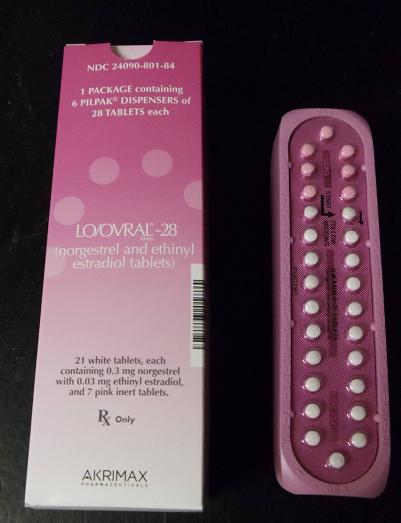
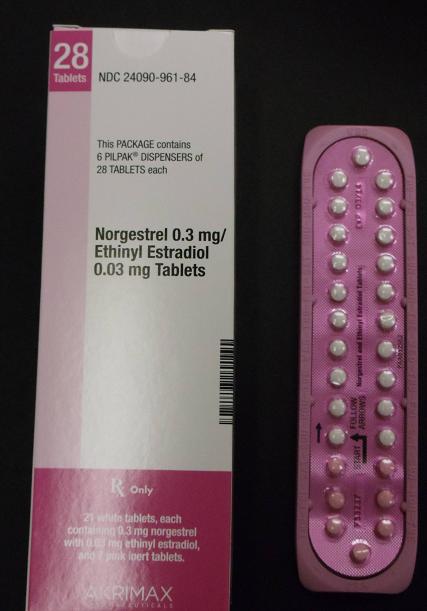
Pfizer Inc. announced today (Jan. 31) that it has voluntarily recalled 14 lots of the birth control pills Lo/Ovral-28, and 14 lots of generic pills labeled Norgestrel and Ethinyl Estradiol tablets.
The recall came after an investigation by Pfizer found that some packs may contain an inexact count of inert or active ingredient tablets, and that the tablets may be out of sequence. The cause was identified and corrected immediately, according to a statement from the company.
As a result of this packaging error, women taking these pills may be without adequate contraception, and at risk for unintended pregnancy, the company said. These packaging defects do not pose any immediate health risks, however, consumers taking pills from the affected lots should begin using a non-hormonal form of contraception immediately. Patients who have the affected product (lot numbers are provided below) should notify their physician and return the product to the pharmacy.
These products are oral contraceptives, indicated for the prevention of pregnancy. These tablets were manufactured and packaged by Pfizer and labeled under the Akrimax Pharmaceuticals brand. This products were distributed to warehouses, clinics and retail pharmacies nationwide, according to the company.
These products are packaged in blister packs, containing 21 tablets of active ingredients and seven tablets of inert ingredients. Correct dosing of this product is important in avoiding an unplanned pregnancy. Correctly packaged blister packs are pictured here.
The company said that any adverse events that may be related to the use of these products should be reported to Akrimax Medical Information at 1-877-509-3935 (8 a.m. to 7p.m. Monday to Friday CST) or to FDA's Med Watch Program either online, by regular mail or by fax.
Online: www.fda.gov/medwatch/report.htm Regular Mail: Use postage-paid, pre-addressed Form FDA 3500 available at: www.fda.gov/MedWatch/getforms.htm. Mail to the address on the pre-addressed form. Fax: 1-800-FDA-0178 This recall is being conducted with the knowledge of the U.S. Food and Drug Administration. Lot numbers of affected packs of Lo/Ovral®-28 (norgestrel and ethinyl estradiol) Tablets and Norgestrel and Ethinyl Estradiol Tablets (generic) follow on the table below:
Get the world’s most fascinating discoveries delivered straight to your inbox.
| NDC | Product | Lot | Expiration | Configuration/Count |
|---|---|---|---|---|
| 24090-801-84 | LO/OVRAL® 28 | E15678 | 08/31/2013 | 6 Pilpacks® of 28 tablets each |
| 24090-801-84 | LO/OVRAL® 28 | E15679 | 08/31/2013 | 6 Pilpacks® of 28 tablets each |
| 24090-801-84 | LO/OVRAL® 28 | E15686 | 08/31/2013 | 6 Pilpacks® of 28 tablets each |
| 24090-801-84 | LO/OVRAL® 28 | E15687 | 01/31/2014 | 6 Pilpacks® of 28 tablets each |
| 24090-801-84 | LO/OVRAL® 28 | E15690 | 01/31/2014 | 6 Pilpacks® of 28 tablets each |
| 24090-801-84 | LO/OVRAL® 28 | E15698 | 01/31/2014 | 6 Pilpacks® of 28 tablets each |
| 24090-801-84 | LO/OVRAL® 28 | E15700 | 02/28/2014 | 6 Pilpacks® of 28 tablets each |
| 24090-801-84 | LO/OVRAL® 28 | E80434 | 07/31/2013 | 6 Pilpacks® of 28 tablets each |
| 24090-801-84 | LO/OVRAL® 28 | E80438 | 08/31/2013 | 6 Pilpacks® of 28 tablets each |
| 24090-801-84 | LO/OVRAL® 28 | F36908 | 02/28/2014 | 6 Pilpacks® of 28 tablets each |
| 24090-801-84 | LO/OVRAL® 28 | F36909 | 02/28/2014 | 6 Pilpacks® of 28 tablets each |
| 24090-801-84 | LO/OVRAL® 28 | F43915 | 03/31/2014 | 6 Pilpacks® of 28 tablets each |
| 24090-801-84 | LO/OVRAL® 28 | F43926 | 03/31/2014 | 6 Pilpacks® of 28 tablets each |
| 24090-801-84 | LO/OVRAL® 28 | F43927 | 03/31/2014 | 6 Pilpacks® of 28 tablets each |
| 24090-961-84 | Norgestrel 0.3 mg/Ethinyl Estradiol 0.03 mg | E15677 | 08/31/2013 | 6 Pilpacks® of 28 tablets each |
| 24090-961-84 | Norgestrel 0.3 mg/Ethinyl Estradiol 0.03 mg | E15704 | 01/31/2014 | 6 Pilpacks® of 28 tablets each |
| 24090-961-84 | Norgestrel 0.3 mg/Ethinyl Estradiol 0.03 mg | E15706 | 01/31/2014 | 6 Pilpacks® of 28 tablets each |
| 24090-961-84 | Norgestrel 0.3 mg/Ethinyl Estradiol 0.03 mg | E80440 | 08/31/2013 | 6 Pilpacks® of 28 tablets each |
| 24090-961-84 | Norgestrel 0.3 mg/Ethinyl Estradiol 0.03 mg | F16388 | 01/31/2014 | 6 Pilpacks® of 28 tablets each |
| 24090-961-84 | Norgestrel 0.3 mg/Ethinyl Estradiol 0.03 mg | F16390 | 02/28/2014 | 6 Pilpacks® of 28 tablets each |
| 24090-961-84 | Norgestrel 0.3 mg/Ethinyl Estradiol 0.03 mg | F22132 | 02/28/2014 | 6 Pilpacks® of 28 tablets each |
| 24090-961-84 | Norgestrel 0.3 mg/Ethinyl Estradiol 0.03 mg | F31330 | 02/28/2014 | 6 Pilpacks® of 28 tablets each |
| 24090-961-84 | Norgestrel 0.3 mg/Ethinyl Estradiol 0.03 mg | F36911 | 03/31/2014 | 6 Pilpacks® of 28 tablets each |
| 24090-961-84 | Norgestrel 0.3 mg/Ethinyl Estradiol 0.03 mg | F36913 | 03/31/2014 | 6 Pilpacks® of 28 tablets each |
| 24090-961-84 | Norgestrel 0.3 mg/Ethinyl Estradiol 0.03 mg | F43924 | 03/31/2014 | 6 Pilpacks® of 28 tablets each |
| 24090-961-84 | Norgestrel 0.3 mg/Ethinyl Estradiol 0.03 mg | F43925 | 03/31/2014 | 6 Pilpacks® of 28 tablets each |
| 24090-961-84 | Norgestrel 0.3 mg/Ethinyl Estradiol 0.03 mg | F43934 | 03/31/2014 | 6 Pilpacks® of 28 tablets each |
| 24090-961-84 | Norgestrel 0.3 mg/Ethinyl Estradiol 0.03 mg | F53238 | 03/31/2014 | 6 Pilpacks® of 28 tablets each |
 Live Science Plus
Live Science Plus