
Firework Colors Enhanced by Elements of Life

Ruchi Shah is a summer science-writing intern for the U.S. National Institute of General Medical Sciences (NIGMS), part of the National Institutes of Health in Bethesda, Maryland. NIGMS supports basic research that increases understanding of biological processes and lays the foundation for advances in disease diagnosis, treatment and prevention. This article was provided by NIGMS to Live Science's Expert Voices: Op-Ed & Insights.
Looking up at the night sky this Fourth of July, you might wonder what gives fireworks their vivid colors — in fact, the bright hues result from chemical elements that are also essential for life.
The human body is made from four key elements: oxygen (65 percent), carbon (18.5 percent), hydrogen (9.5 percent) and nitrogen (3.3 percent), for a total mass of about 96 percent. These elements are found in our body's most abundant and important molecules, including water, proteins and DNA. A dozen or so other elements — mostly metals — make up the remaining 4 percent. Present in minuscule amounts, these elements are involved in everything from transporting oxygen and releasing hormones to regulating blood pressure and maintaining bone strength. They also give a burst of color when they are added to a fireworks recipe.
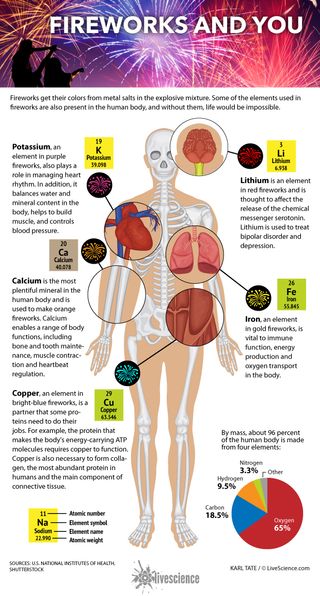
Calcium is the most plentiful mineral in the human body and is needed to make orange fireworks. It's crucial for a range of bodily functions including bone and tooth maintenance, muscle contraction, hormone secretion, blood clotting and heartbeat regulation.
Potassium, which helps create purple fireworks, also plays a role in managing heart rhythm. In addition, it balances water and mineral content in the body, helps to build muscle and controls blood pressure. [The Fireworks Inside Us All ]
Copper, found in bright blue fireworks, is the sidekick that some proteins need to do their jobs. For example, the protein that makes the body's energy-carrying ATP molecules requires copper to function. Copper is also necessary to form collagen, the most abundant protein in humans and the main component of connective tissue.
Iron, used to make gold fireworks, is vital to immune function, energy production and oxygen transport in the body. Hemoglobin, the protein that gives blood its red color, needs iron to carry oxygen from the lungs to the rest of the body.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.

Lithium helps produce red fireworks and is thought to affect the release of the chemical messenger serotonin. Lithium has been used for decades to treat mood disorders.
For details about other elements’ roles in the body, see Metals in Health and Disease.
Follow all of the Expert Voices issues and debates — and become part of the discussion — on Facebook, Twitter and Google+. The views expressed are those of the author and do not necessarily reflect the views of the publisher. This version of the article was originally published on Live Science.
Most Popular


