Where do electrons get energy to spin around an atom's nucleus?
Electrons were once thought to orbit a nucleus much as planets orbit the sun. That picture has since been obliterated by modern quantum mechanics.

An atom is best visualized as a tight, dense nucleus surrounded by buzzing, orbiting electrons. This picture immediately leads to a question: How do electrons keep whirling around the nucleus without ever slowing down?
This was a burning question in the early 20th century, and a search for the answer ultimately led to the development of quantum mechanics itself.
In the early 20th century, after countless experiments, physicists were just beginning to put together a coherent picture of the atom. They realized that each atom had a dense, heavy, positively charged nucleus surrounded by a cloud of tiny, negatively charged electrons. With that general picture in mind, their next step was to create a more detailed model.
Related: Weird 'gravitational molecules' could orbit black holes like electrons swirling around atoms
In the earliest attempts at this model, scientists took their inspiration from the solar system, which has a dense "nucleus" (the sun) surrounded by a "cloud" of smaller particles (the planets). But this model introduced two significant problems.
For one, a charged particle that accelerates emits electromagnetic radiation. And because electrons are charged particles and they accelerate during their orbits, they should emit radiation. This emission would cause the electrons to lose energy and quickly spiral in and collide with the nucleus, according to the University of Tennessee at Knoxville. In the early 1900’s physicists estimated that such an inward spiral would take less than one-trillionth of a second, or a picosecond. Since atoms obviously live longer than a picosecond, this wasn't going to work.
A second, more subtle issue had to do with the nature of the radiation. Scientists have known that atoms emit radiation, but they do so at very discrete, specific frequencies. An orbiting electron, if it followed this solar system model, would instead emit all sorts of wavelengths, contrary to observations.
Get the world’s most fascinating discoveries delivered straight to your inbox.
The quantum fix
Famed Danish physicist Niels Bohr was the first person to propose a solution to this issue. In 1913, he suggested that electrons in an atom couldn't just have any orbit they wanted. Instead, they had to be locked into orbits at very specific distances from the nucleus, according to the Nobel Prize citation entry for his subsequent award. In addition, he proposed that there was a minimum distance an electron could reach and that it could move no closer to the nucleus.
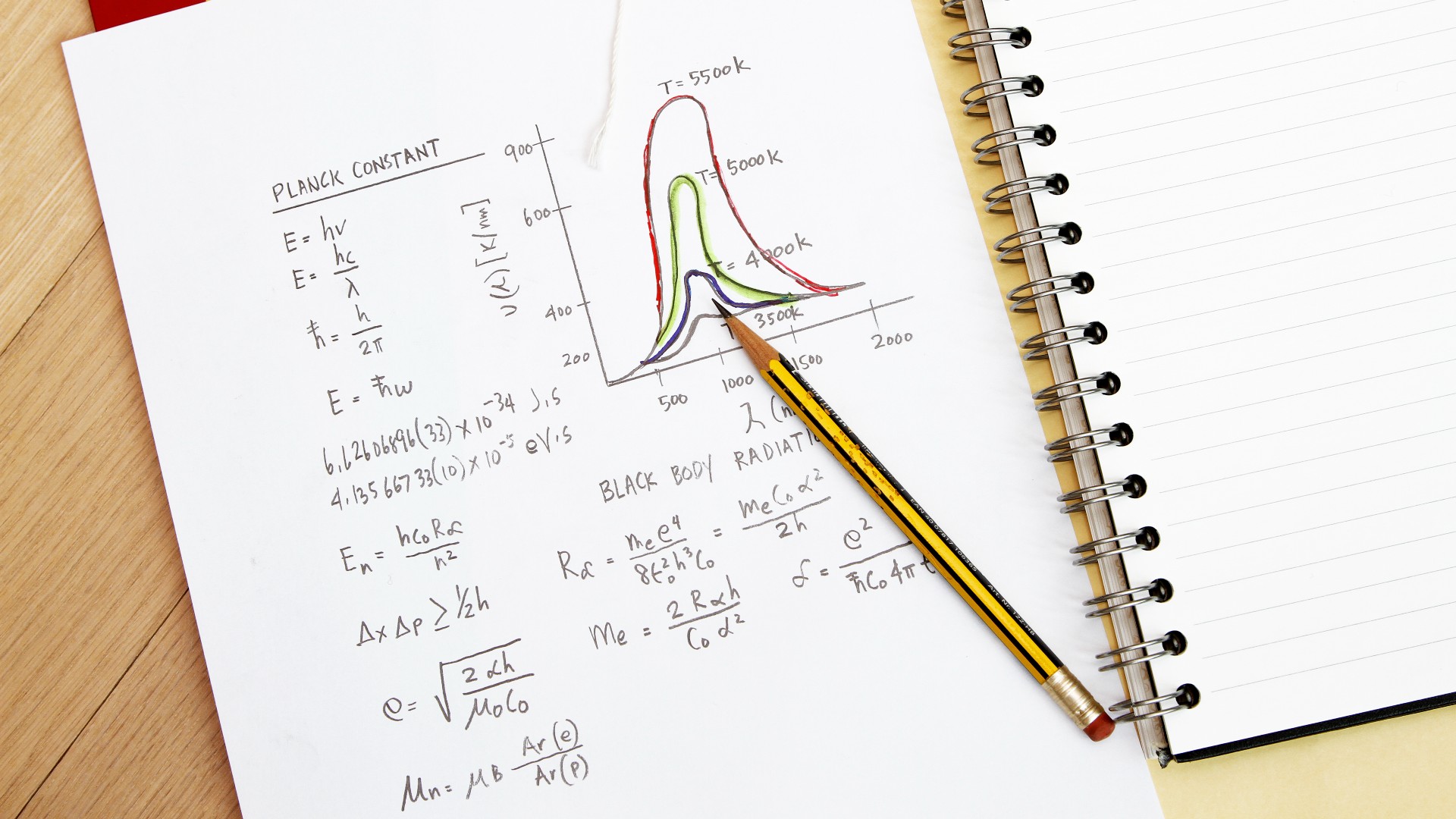
He didn't just pull these ideas out of a hat. A little over a decade before, German physicist Max Planck had proposed that the emission of radiation might be "quantized," meaning an object could only absorb or emit radiation in discrete chunks, and not have any value it wanted, according to the HyperPhysics reference page at Georgia State University. But the smallest size of these discrete chunks was a constant, which came to be known as Planck's constant. Prior to this, scientists thought such emissions were continuous, meaning particles could radiate at any frequency.
Planck's constant has the same units as angular momentum, or the momentum of an object moving in a circle. So Bohr imported this idea to electrons orbiting a nucleus, saying that the smallest possible orbit of an electron would equal the angular momentum of exactly one Planck constant. Higher orbits could have twice that value, or three times, or any other integer multiple of the Planck constant, but never any fraction of it (so not 1.3 or 2.6 and so forth).
It would take the full development of quantum mechanics to understand why electrons had such a minimum orbit and clearly defined higher orbits. Electrons, like all matter particles, behave as both particles and waves. While we might imagine an electron as a tiny planet orbiting the nucleus, we can just as easily imagine it as a wave wrapping around that nucleus.
Waves in a confined space have to obey special rules. They can't just have any wavelength; they must be made out of standing waves that fit inside the space. It's just like when someone plays a musical instrument: If you pin down the ends of a guitar string, for example, only certain wavelengths will fit, giving you the separate notes. Similarly, the electron wave around a nucleus has to fit, and the nearest orbit for an electron to a nucleus is given by the first standing wave of that electron.
Future developments in quantum mechanics would continue to refine this picture, but the basic point remains: An electron can't get any closer to a nucleus because its quantum mechanical nature won't let it take up any less space.
Adding up the energies
But there's a completely different way to examine the situation that doesn't rely on quantum mechanics at all: Just look at all the energies involved. An electron orbiting a nucleus is electrically attracted to the nucleus; it's always being pulled closer. But the electron also has kinetic energy, which works to send the electron flying away.
For a stable atom, these two are in balance. In fact, the total energy of an electron in orbit, which is a combination of its kinetic and potential energies, is negative. That means you have to add energy to the atom if you want to remove the electron. It's the same situation with the planets in orbit around the sun: To remove a planet from the solar system, you'd have to add energy to the system.
One way to view this situation is to imagine an electron "falling" toward a nucleus, attracted by its opposite electric charge. But because of the rules of quantum mechanics, it can't ever reach the nucleus. So it gets stuck, forever orbiting. But this scenario is allowed by physics, because the total energy of the system is negative, meaning it's stable and bound together, forming a long-lasting atom.
Originally published on Live Science on Jan. 21, 2011 and rewritten on June 22, 2022.

Paul M. Sutter is a research professor in astrophysics at SUNY Stony Brook University and the Flatiron Institute in New York City. He regularly appears on TV and podcasts, including "Ask a Spaceman." He is the author of two books, "Your Place in the Universe" and "How to Die in Space," and is a regular contributor to Space.com, Live Science, and more. Paul received his PhD in Physics from the University of Illinois at Urbana-Champaign in 2011, and spent three years at the Paris Institute of Astrophysics, followed by a research fellowship in Trieste, Italy.