Infertility Genes Could Lead to Male Contraception
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
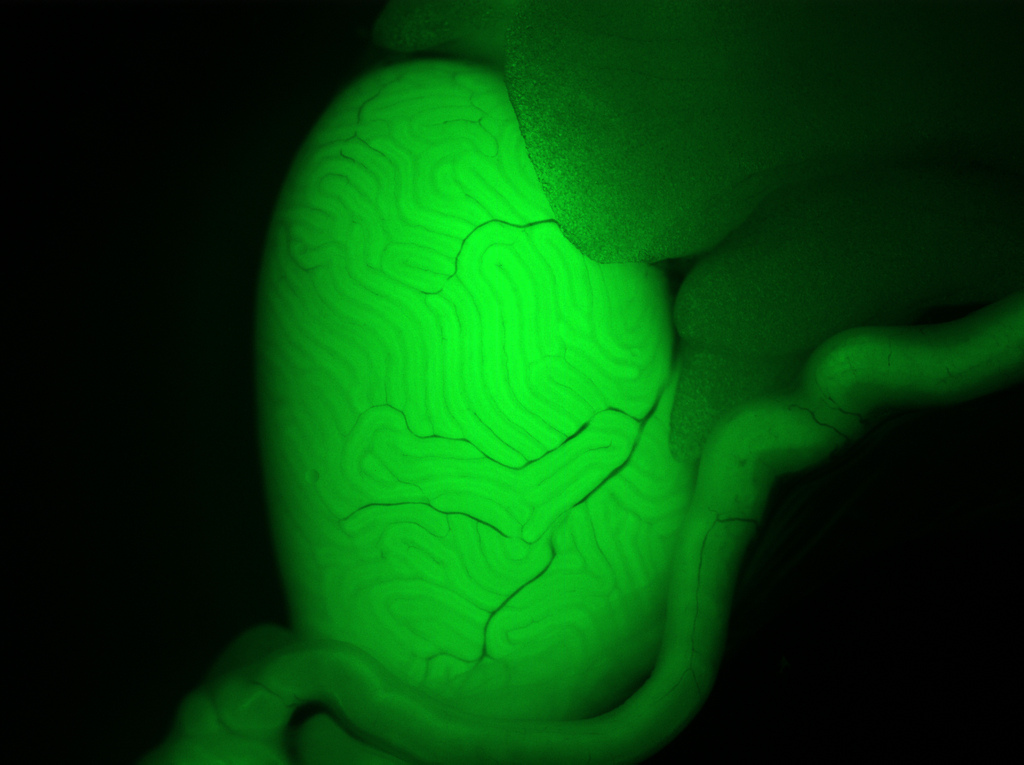
Genes in charge of making sperm cells may be the key to understanding male infertility and even developing male contraception, two new studies indicate.
With this new information in hand, scientists say a male non-hormonal contraceptive may be just five to 10 years away.
Infertility remains a sensitive topic, and about 25 percent of cases remain unexplained. Getting a better grasp on the genetic causes of infertile men could lead to better treatments, the researchers say.
"The irony is that it is the fact that these men don't have children that makes standard family-pedigree analysis very challenging, and as such it has historically been extremely difficult to identify genetic causes of specific cases of male infertility," Lee Smith of the University of Edinburgh in Scotland, who studies male infertility,told LiveScience.
Fertility genes
The results, published today (May 24) in the American Journal of Human Genetics, are based on a study of the genetics of a group of men who belong to a religious group called the Hutterites, pacifist, self-sufficient colonists similar to the Amish.
"Hutterites [forbid] contraception and uniformly desire large families, providing an outstanding population in which to study the genetics of normal human fertility," study researcher Carole Ober of the University of Chicago explained in a statement. [Birth Control Quiz: Test Your Contraception Knowledge]
Get the world’s most fascinating discoveries delivered straight to your inbox.
The researchers studied Hutterite men with one or more children, taking both family size and birth rate into consideration. They uncovered more than 40 genetic regions that influence how fertile a Hutterite man is, and then compared these with genetic sequences from a sample of Chicago men, in which nine of these same regions seemed to impact fertility.
"We do expect that genes identified … in the Hutterites will be relevant fertility genes in other populations, especially those that were also associated with sperm parameters in our validation studies" of the Chicagoans, Ober told LiveScience.
The next step, Smith said, is to use animal models to figure out the functions of these fertility-impacting genes. In another study, also published today, in the journal PLoS Genetics, Smith does just that.
Immature sperm
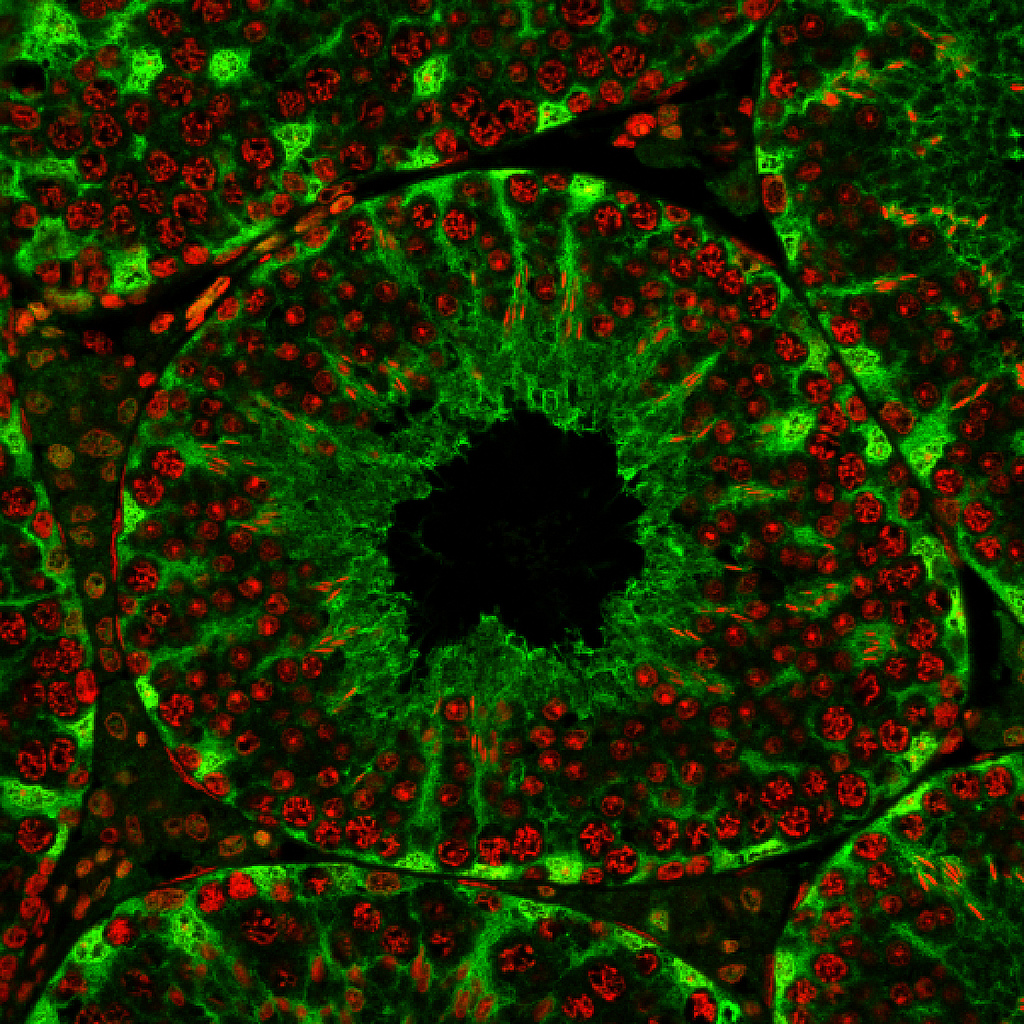
Smith and his colleagues fed drugs to mice that gave them various genetic mutations. Next, to pinpoint genes related to fertility, the researchers identified the infertile mice of the bunch. They then traced the infertility back to the gene mistake that caused it and looked at its effect on the mouse's sperm cells.
The researchers identified one specific gene, called Katnal1, that is crucial for sperm formation. Without the protein created from this gene, mouse sperm can't mature in the testes. These immature sperm are infertile.
The researchers think the same genetic link to infertility may be found in humans; if they can find a drug to manipulate Katnal1 levels in men, or do so permanently using gene therapy, the result might work as a non-hormonal contraceptive. The findings also may explain some cases of infertility: Perhaps the man has a natural mutation that messes Katnal1 up.
"Identification of genetic mutations associated with infertility that affect the supporting cells (and not the sperm themselves) could lead to personalized gene therapy (replacement of faulty genes) for male infertility within five to 10 years," Smith said. "All of the components have been tested and validated in rodent models. Likewise a genetic vasectomy … could also be available within five to 10 years."
You can follow LiveScience staff writer Jennifer Welsh on Twitter, on Google+ or on Facebook. Follow LiveScience for the latest in science news and discoveries on Twitter and on Facebook.
Jennifer Welsh is a Connecticut-based science writer and editor and a regular contributor to Live Science. She also has several years of bench work in cancer research and anti-viral drug discovery under her belt. She has previously written for Science News, VerywellHealth, The Scientist, Discover Magazine, WIRED Science, and Business Insider.
 Live Science Plus
Live Science Plus