Amoeba Causes Disease That Spreads in Unconventional Way
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
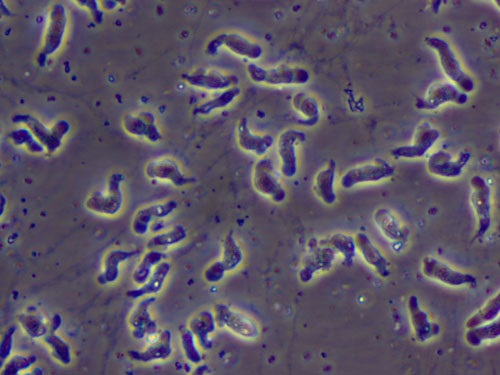
A rare heat-loving amoeba caused an infection that killed a 9-year-old girl in Kansas on July 9, and new research may help shed light on how it and other similar infectious diseases spread.
The amoeba, Naegleria fowleri, and the infection it causes, belong to a class of infectious diseases called sapronoses. Conventional infectious diseases spread from contact between people or other animals, but sapronoses are different — the infections they cause come from tiny organisms that are living in water or soil rather than inhabiting a living host.
For instance, brain-eating amoebas lurk in warm bodies of water, where they can find unsuspecting hosts, entering the host's brain through the nose. The Kansas girl likely picked up the rare parasite after swimming in one of several local lakes, news reports suggest. The infection caused by the amoeba is rare — only 132 cases have been reported in the United States since 1962, according to the Kansas Department of Health. [7 Devastating Infectious Diseases]
These kinds of diseases are poorly studied, because they emerge sporadically. Now, researchers have created a mathematical model to illustrate the differences between the spread of more conventional infectious diseases, like HIV and the flu, and the spread of sapronotic diseases, like anthrax (caused by Bacillus anthracis), Legionnaire's disease and primary amebic meningoencephalitis (the infection that killed the Kansas girl who picked up the amoeba).
"Sapronoses do not follow the rules of infectious diseases that are transmitted from host to host," Armand Kuris, a professor in the University of California, Santa Barbara's Department of Ecology, Evolution, and Marine Biology, said in a statement. "They are categorically distinct from the way we think infectious diseases should operate."
For a conventional infectious disease to spread successfully, it must infect a new host before its current host dies or recovers (and so kills the "bug"). This so-called "host-density threshold theorem" advances the idea that a disease epidemic can only happen if the disease can infect more than one host before it kills its current host, or the host recovers.
The entire premise behind human efforts to fight infectious diseases is based on the threshold theorem. If enough people are vaccinated, then the number of potential new hosts drops off so that epidemics are prevented. However, sapronotic infectious diseases don't fit the threshold theorem, as they don't depend on a population for survival. They lie in wait for an opportunity to infect a host.
Get the world’s most fascinating discoveries delivered straight to your inbox.
The larger a sapronotic disease population gets, the more likely it is that a host will become infected, according to the researchers' model. But unlike conventional infectious diseases, sapronotic diseases do not require a minimum number of hosts to survive, Kuris explained. Other infectious diseases can't survive in a population if the population gets too low — the disease needs a living host, so when it runs out of people or other animals to infect, it eventually dies out. These diseases can be eradicated by isolating and treating those infected. A sapronotic disease is different, because even after it has been eradicated from a population, the disease can emerge again later from the water or soil in which it lives.
"Controlling sapronoses is not about treating infected hosts,” the researchers write in the August issue of the journal Trends in Parasitology. "Whereas treating infected individuals will remain the most important and urgent response to combat sapronoses, controlling them requires reducing contact with, or sterilizing or otherwise altering, the environments where they proliferate."
The researchers randomly chose 150 bacteria, protozoans and fungi that cause diseases in humans and discovered that about one-third were sapronotic. The researchers noted the significance of this number: almost 97 percent of the fungi were sapronotic.
As humans continue to colonize more and more areas of the world, it is likely they will become exposed to more sapronoses, the researchers write in the paper. More research is needed to better understand how the diseases spread.
Follow Kelly Dickerson on Twitter. Follow us @livescience, Facebook & Google+. Original article on Live Science.

 Live Science Plus
Live Science Plus










