New Malaria Vaccine Made from Mosquito Spit
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
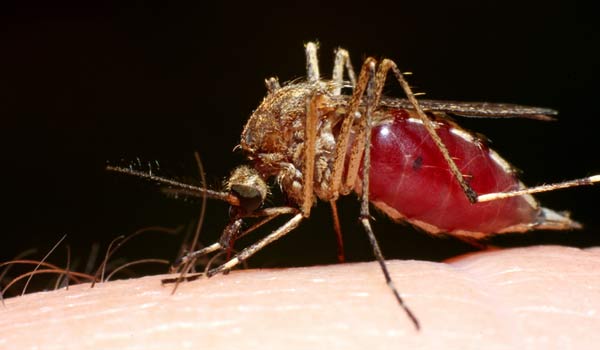
One of the more promising avenues of creating a vaccine for malaria involves going inside mosquitoes' bodies — typically the source of the disease's spread — to develop the key component of the vaccine.
Research published online in the journal Science today (Sept. 8) shows the barriers and future direction for that vaccine. A clinical trial of such a vaccine showed that it was safe, but that it didn't confer immunity to enough of the study participants.
"What we believe is needed is a highly effective malaria vaccine. To our minds, that means 80 percent protective, preferably 90 percent protective, for at least six months, preferably for several years," said study researcher Stephen L. Hoffman, a medical doctor who founded the company Sanaria Inc. in 2002 with the aim of creating a malaria vaccine.
While the new study showed only 5 percent of participants developed immunity, further experiments conducted on mice and rhesus macaques showed that a different method of administering the vaccine — into the bloodstream, rather than into the skin or body fat, as was done with the human volunteers — might produce the desired results.
Using mosquitoes to make a vaccine
Creating a malaria vaccine within the bodies of mosquitoes is an idea that has shown promise since the 1970s, when it was first rigorously studied. But getting from concept to a safe, effective vaccine was seen as impractical, Hoffman said.
Malaria is spread via mosquitoes: When an infected insect bites, it passes the malaria parasite, called Plasmodium falciparum, into human blood. When the parasite reaches the liver, it reproduces, spreads throughout the body and causes malaria.
Get the world’s most fascinating discoveries delivered straight to your inbox.
The mosquito-based vaccine uses infected mosquitoes that have been irradiated. As a result, once the parasites reach the liver, they do not reproduce or cause disease. However, their presence still triggers the body's immune system to build a defense against infection.
Even if the concept for this vaccine sounds simple, making it happen in real life is not.
For one, mosquitoes tend to carry diseases besides malaria. And transmission via mosquito bite is also a problem, given the sheer number of such bites needed to deliver immunity. A 1973 trial showed 819 mosquito bites were needed to confer the desired immunity to malaria.
In the new study, the researchers raised their own mosquitoes and kept them free of disease except for the malaria parasite. After irradiation, they extracted the insects' salivary glands to make the vaccine.
Only two out of the 40 vaccinated people developed immunity to malaria, prompting Sanaria to contact National Institutes of Health researchers about experimenting with a different method of vaccine delivery.
In the animal experiments using the intravenous vaccine, 71 percent to 100 percent developed immunity.
The problem remains of whether this will work in people – and of finding an efficient way of administering the vaccine to large populations. Worldwide, there are 300 million malaria cases and 1 million deaths each year, the study said.
But the approach shows promise, said Dr. Anna Durbin, an associate professor of international health at the Bloomberg School of Public Health at Johns Hopkins University.
"I don't think I can stress enough how difficult the concept of mass producing such a vaccine is, and how the company has done that," Durbin said.
Not effective enough
Going forward, Durbin said, a clinical trial is needed in which the vaccine is injected intravenously.
An intravenous injection would be a first for a vaccine – most are injected into the muscle, with a few injected into subcutaneous fat or the skin – and might make mass immunization campaigns difficult, Durbin said.
Robert Seder, who led the NIH researchers in the animal experiments, said that even if an intravenous vaccine cannot be scaled up for mass immunization in countries where malaria is endemic, there would be a market among some Americans for it. They would include military recruits going to Africa; students or other visitors going there; public health workers and oil rig workers.
"They could get intravenous [vaccines] rather than having to take medicine for years and years," said Seder, referring to the available malaria medicines, which must be taken daily or weekly as long as a person is in an infected region.
Other malaria vaccines in development have also failed to meet the 80-to-90 percent protection mark. In the July edition of the journal Lancet Infectious Diseases, a malaria vaccine by GlaxoSmithKline now entering Phase III trials (the final trials before approval) reported an efficacy below 60 percent in Phase II trials in children.
The mechanism of other malaria vaccines relies on a single protein from the parasite, and while these vaccines do not fully prevent infection, they appear to alter the course of the disease and reduce its severity, Seder said.
"This is a unique vaccine," Seder said. "It's an irradiated parasite – never been done before."
The NIH will begin intravenous human vaccine trials this fall.
"There's still going to be those problems, but we're mowing down the hurdles one by one," Seder said. "I'm cautiously optimistic that this is something that will be much better, but we have to see."
This story was provided by MyHealthNewsDaily, a sister site to LiveScience. Follow MyHealthNewsDaily on Twitter @MyHealth_MHND. Find us on Facebook.
 Live Science Plus
Live Science Plus






